Phospholipases PLBD1 and PLBD2: Difference between revisions
Tomemerald (talk | contribs) |
Tomemerald (talk | contribs) |
||
| Line 2: | Line 2: | ||
(to be continued shortly) | (to be continued shortly) | ||
=== | === Conservation at critical sites === | ||
[[Image:PLBD2activeSiteComp.png|left]] | [[Image:PLBD2activeSiteComp.png|left]] | ||
| Line 10: | Line 10: | ||
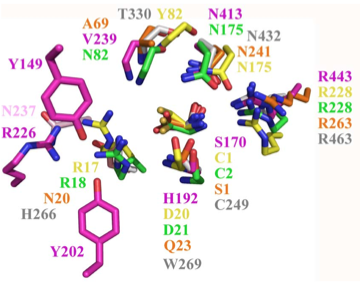
(adapted from Fig 6 of [http://www.ncbi.nlm.nih.gov/pmc/articles/pmid/19706171 Lakomek et al. BMC Struct Biol.2009;9:56:)] | (adapted from Fig 6 of [http://www.ncbi.nlm.nih.gov/pmc/articles/pmid/19706171 Lakomek et al. BMC Struct Biol.2009;9:56:)] | ||
* (*) * * | * (*) * * | ||
PLBD2 phospholipase B-like gray 3FGR C249 H266 W269 T330 N432 R463 | PLBD2 phospholipase B-like gray 3FGR C249 H266 W269 T330 N432 R463 mouse numbering | ||
PLBD1 phospholipase B-like .... pred | PLBD1 phospholipase B-like .... pred C228 H245 W248 T303 N402 R433 [http://swissmodel.expasy.org/repository/?pid=smr03&uid=&token=&query_1_input=Q8VCI0&zid=async SwissModel] | ||
Cephalosporin acylase pink 1OQZ S170 .... H192 .... N413 R443 | Cephalosporin acylase pink 1OQZ S170 .... H192 .... N413 R443 | ||
Conjugated bile acid hydrolase green 2BJF C2 .... D21 .... N175 R228 | Conjugated bile acid hydrolase green 2BJF C2 .... D21 .... N175 R228 | ||
| Line 29: | Line 29: | ||
[[Image:PLBD1colored.png|left]] | [[Image:PLBD1colored.png|left]] | ||
<br clear ="all"> | <br clear ="all"> | ||
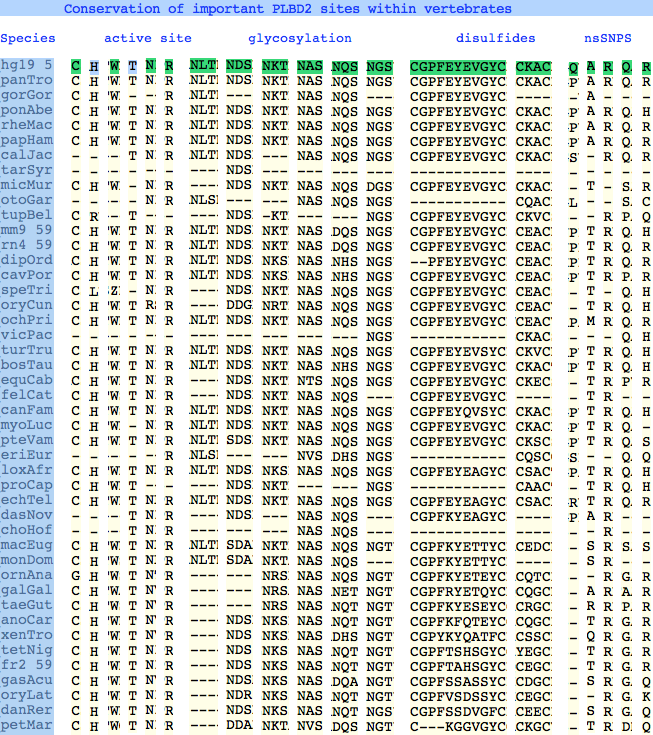
The six residues of PLBD2 associated with the active site are completely conserved within vertebrates to within genomic sequencing error. These same six residues are also completely conserved within PLBD1. Indeed 3 of the residues are conserved in the broader class of NTN hydrolases. | |||
This is perhaps unsurprising since the active site was established a billion years earlier in the bacterial ancestor. However if PLBD2 and PLBD1 have different substrates, this establishes that these six residues are insufficient to distinguish the two active sites. Note H266 and T330 do not contribute their side chain, leaving them and W269 to separate phospholipases from the other NTN hydrolases. | |||
The glycosylation sites are surprisingly conserved both within and between PLBD2 and PLBD1. Some of the motifs may be either recently acquired within later vertebrates or spurious glycosylation motifs with N and D both acceptable (or similar small amino acids). Glycosylation is important in targeting of lysosomal proteins, more so than in generic endoplasmic reticulum proteins where motifs are often poorly conserved (as in sulfatases). | |||
The known human SNPs of PLBD2 are in some cases quite radical substitutions in terms of both physical qualities of the substituted amino acid and the degree of observed phylogenetic conservation at that site. These likely result in unstable and/or inactive enzyme. Both enzymes are autosomal so compensation might occur in the recessive state, or alternately, PLBD2 and PLBD1 could fill for each other to some extent. In either case, lysosomal storage disease might not be clinically observable. | |||
Here Q54P may actually be a mutation in the reference sequence individual (with the SNP representing wildtype) as proline is quite well conserved throughout mammals. In A204V, valine is quite a bulky substituent for a site normally restricted to small amino acids; R354C is definitely a serious mutation, no doubt attributable to a CpG hotspot; Q521K appears milder as does R524C. | |||
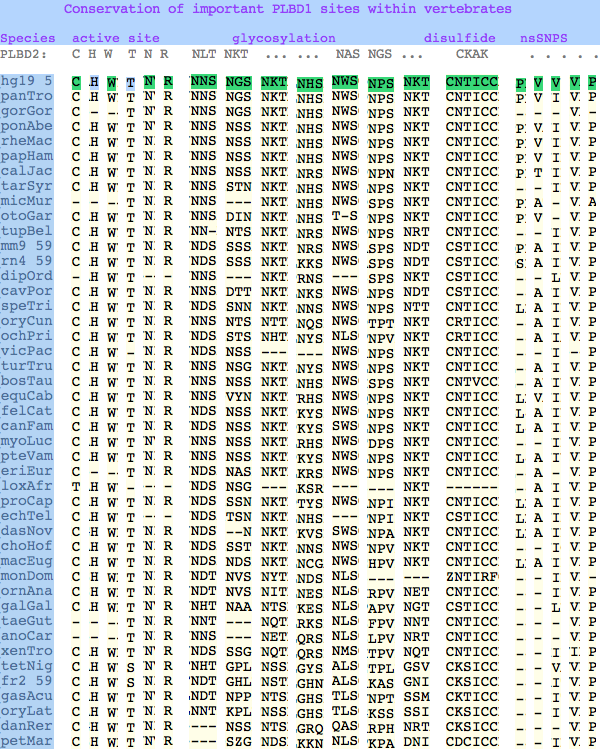
The known human SNPs of PLBD1 can be analyzed similarlly. P26Q and V30L may be inconsequential as they occur in the rather unconstrained primary sequence of the N-terminus; V265I occurs at an ILV reduced alphabet; V377A and P534A are much more serious despite the aliphatic nature of alanine and likely give rise to dysfunctional protein. | |||
[[Image:PLDB2consSites.png|left]] | [[Image:PLDB2consSites.png|left]] | ||
<br clear ="all"> | <br clear ="all"> | ||
Revision as of 12:52, 26 October 2010
Introduction
(to be continued shortly)
Conservation at critical sites
Structural superposition of active sites from five NTN hydrolases showing conserved side chains (*) and relevant main chains (....) (adapted from Fig 6 of Lakomek et al. BMC Struct Biol.2009;9:56:) * (*) * * PLBD2 phospholipase B-like gray 3FGR C249 H266 W269 T330 N432 R463 mouse numbering PLBD1 phospholipase B-like .... pred C228 H245 W248 T303 N402 R433 SwissModel Cephalosporin acylase pink 1OQZ S170 .... H192 .... N413 R443 Conjugated bile acid hydrolase green 2BJF C2 .... D21 .... N175 R228 Penicillin V acylase yellow 3PVA C1 .... D20 .... N175 R228 Penicillin G acylase orange 1K5S S1 .... Q23 .... N241 R263 Human SNPs resulting in amino acid substitions: PLBD2: PLBD1: Q54P rs7965471 P26Q rs1141509 A204V rs12231990 V30L rs12296104 R354C rs56935204 V265I rs7957558 Q521K rs17852787 V377A rs2287541 R524C rs12425042 P534A rs1600
The six residues of PLBD2 associated with the active site are completely conserved within vertebrates to within genomic sequencing error. These same six residues are also completely conserved within PLBD1. Indeed 3 of the residues are conserved in the broader class of NTN hydrolases.
This is perhaps unsurprising since the active site was established a billion years earlier in the bacterial ancestor. However if PLBD2 and PLBD1 have different substrates, this establishes that these six residues are insufficient to distinguish the two active sites. Note H266 and T330 do not contribute their side chain, leaving them and W269 to separate phospholipases from the other NTN hydrolases.
The glycosylation sites are surprisingly conserved both within and between PLBD2 and PLBD1. Some of the motifs may be either recently acquired within later vertebrates or spurious glycosylation motifs with N and D both acceptable (or similar small amino acids). Glycosylation is important in targeting of lysosomal proteins, more so than in generic endoplasmic reticulum proteins where motifs are often poorly conserved (as in sulfatases).
The known human SNPs of PLBD2 are in some cases quite radical substitutions in terms of both physical qualities of the substituted amino acid and the degree of observed phylogenetic conservation at that site. These likely result in unstable and/or inactive enzyme. Both enzymes are autosomal so compensation might occur in the recessive state, or alternately, PLBD2 and PLBD1 could fill for each other to some extent. In either case, lysosomal storage disease might not be clinically observable.
Here Q54P may actually be a mutation in the reference sequence individual (with the SNP representing wildtype) as proline is quite well conserved throughout mammals. In A204V, valine is quite a bulky substituent for a site normally restricted to small amino acids; R354C is definitely a serious mutation, no doubt attributable to a CpG hotspot; Q521K appears milder as does R524C.
The known human SNPs of PLBD1 can be analyzed similarlly. P26Q and V30L may be inconsequential as they occur in the rather unconstrained primary sequence of the N-terminus; V265I occurs at an ILV reduced alphabet; V377A and P534A are much more serious despite the aliphatic nature of alanine and likely give rise to dysfunctional protein.
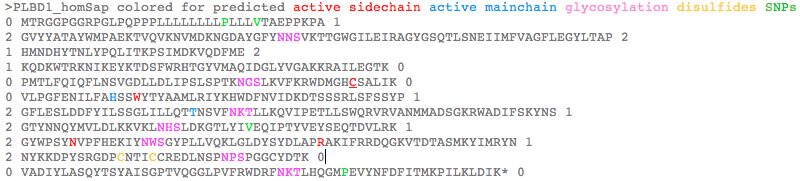
PLBD1 reference sequences
>PLBD1_homSap Homo sapiens (human) FLJ22662 PMID: 19019078,20093120 0 MTRGGPGGRPGLPQPPPLLLLLLLLPLLLVTAEPPKPA 1 2 GVYYATAYWMPAEKTVQVKNVMDKNGDAYGFYNNSVKTTGWGILEIRAGYGSQTLSNEIIMFVAGFLEGYLTAP 2 1 HMNDHYTNLYPQLITKPSIMDKVQDFME 2 1 KQDKWTRKNIKEYKTDSFWRHTGYVMAQIDGLYVGAKKRAILEGTK 0 0 PMTLFQIQFLNSVGDLLDLIPSLSPTKNGSLKVFKRWDMGHCSALIK 0 0 VLPGFENILFAHSSWYTYAAMLRIYKHWDFNVIDKDTSSSRLSFSSYP 1 2 GFLESLDDFYILSSGLILLQTTNSVFNKTLLKQVIPETLLSWQRVRVANMMADSGKRWADIFSKYNS 1 2 GTYNNQYMVLDLKKVKLNHSLDKGTLYIVEQIPTYVEYSEQTDVLRK 1 2 GYWPSYNVPFHEKIYNWSGYPLLVQKLGLDYSYDLAPRAKIFRRDQGKVTDTASMKYIMRYN 1 2 NYKKDPYSRGDPCNTICCREDLNSPNPSPGGCYDTK 0 0 VADIYLASQYTSYAISGPTVQGGLPVFRWDRFNKTLHQGMPEVYNFDFITMKPILKLDIK* 0 >PLBD1_braFlo Branchiostoma floridae (lancelet) XM_002595538 0 MEGRACRSCRLHHLSAVFLLFLVTIAA 1 2 GAEIQATAYLQAQGKVQVKLGVLDKQNGDAVATYDDR 2 1 LTENGWGVLNVVSGFGPKKLSDNDIMYLAGYLEGVLTQE 2 1 RIYQHYLNLYGIFFMGKSEDLVGK 0 0 VKKFYTAQDTWVRAQVKQSTDPVMKHLSYILSQYDGLVKGYNDN 0 0 LFPHVSFFQKLDIFAFQLLNGNGDTFDIIPAVNPSSRPDFSNMSRVEIDDWVSAHSHCSALVK 0 0 VLGAYENVYMSHSSWFNYAATMRIYKHYNFNIANPATATRKMSFSSYP 1 2 GYLESLDDFYLMDSGLVMLQTTNNVFNGTLYDLVKPESILAWQRVRTANMLARNGDQWGAIMNVHNS 1 2 GTYNNQYMIIDLNLIELGKTIHDGALYVVEQIPGLVMSADQTDILRA 1 2 GYWPSYNIPFYEKVYNLSGYPEFAKSQGLDYTYQLAPRAKIFRRDAGKVKDMESMKAIMRYN 1 2 DYLHDPYSKGNPCSAICCRKDLAKVGAKPDGCYDTK 0 0 VSDYYLARNLTSFAINGPTLGTGLEPFSWSDKFKISHIGLPKVYNFSFVTMTPAEL* 0
PLBD2 reference sequences
>PLBD2_homSap Homo sapiens (human) PMID: 19706171,19237744,17007843 0 MVGQMYCYPGSHLARALTRALALALVLALLVGPFLSGLAGAIPAPGGRWARDGQVPPASRSRSVLLDVSAGQLLMVDGRHPDAVAWANLTNAIRETG 2 1 WAFLELGTSGQYNDSLQAYAAGVVEAAVSEE 0 0 LIYMHWMNTVVNYCGPFEYEVGYCERLKSFLEANLEWMQEEMESNPDSPYWHQ 0 0 VRLTLLQLKGLEDSYEGRVSFPAGKFTIKPLGFL 2 1 LLQLSGDLEDLELALNKTKIKPSLGSGSCSALIKLLPGQSDLLVAHNTWNNYQHMLRVIKKYWLQFREGPW 1 2 GDYPLVPGNKLVFSSYPGTIFSCDDFYILGSGL 0 0 VTLETTIGNKNPALWKYVRPRGCVLEWVRNIVANRLASDGATWADIFKRFNSGT 2 1 YNNQWMIVDYKAFIPGGPSPGSRVLTILEQIP 2 1 GMVVVADKTSELYQKTYWASYNIP 2 1 SFETVFNASGLQALVAQYGDWFSYDGSPRAQIFRRNQSLVQDMDSMVRLMR 2 1 YNDFLHDPLSLCKACNPQPNGENAISARSDLNPANGSYPFQALRQRSHGGIDVK 0 0 VTSMSLARILSLLAASGPTWDQVPPFQWSTSPFSGLLHMGQPDLWKFAPVKVSWD* 0 >PLBD2_braFlo Branchiostoma floridae (lancelet) XM_002612057 0 MAACRNIFCGRMLSCLLLFSFVFSAVSDGSKLASVRYDEAAKTYQITDKLDPSAAAWANFTDRISSTG 2 1 WSFLTVTTNEKYDDSVQAYAAGLVEGYLTRD LMYNHWLNTVGAAFCSSRSAFCKNLESFLKTNLAWMQEQIQASGDTDDYWHQ 0 0 VKLTLQQLSGLDDGYNDDPRQPSLDINPFGFL 2 1 IFQIGGDMEDLQEALKDKDSHRVLGSGSCSALVKLLPGNADLLVAHDTWDTFQSMLRIIKKYQFPFKLGGKK 1 2 GEDKIPGHTVSFSSYPGVIYSGDDFYITSASL 0 0 VAQETTIGNSNPALWKYVQPQGQVLEWLRNIVANRLANKAMDWATIFKKYNSGT 2 1 YNNQWMIVDYKTFTPNKDLPEKGLLVVLEQLP 2 1 GMVMMDDVTSVLAKQAYWPSYNSP 2 1 YFEKIFNTSGLPAMVEKYGDWFSYEHTPRANIFRRDHGKVTDISSMIKLMR 2 1 YNDFQNDPLSKCDCTPPYSAENAISARSDLNPANGTYPFSALQHRCHGGTDMK 0 0 MTSYSMHESHQMMAVSGPTHDQQQPFQWSTSDYDKQFYHLGHPDLFNFDPIHVIWFDQSDN* 0 >PLBD2_droMel Drosophila melanogaster (fruitfly) U57314 retinal lamina neuron ancestor (lama) PMID: 16077094,8892229 MERPEYDGTYCATALWTKQVGFQIENWKQQNDLVNIPTGVGRICYKDSVYENGWAQIEVETQRTYPDWVQAYAAGMLEGSLTWRNIYNQWSNTISSSCERDESTQKFCGWLRDLLTTNYHRLKRQT EKAENDHYWHQLHLFITQLEGLETGYKRGASRARSDLEEEIPFSDFLLMNAAADIQDLKIYYENYELQNSTEHTEEPRTDQPKNFFLPSATMLTKIVQEEESPQVLQLLFGHSTAGSYSSMLRIQK RYKFHYHFSSKLRSNTVPGVDITFTGYPGILGSTDDFYTIKGRHLHAIVGGVGIKNENLQLWKTVDPKKMVPLVARVMAANRISQNRQTWASAMSRHPFTGAKQWITVDLNKMKVQDNLYNVLEGD DKHDDAPVVLNEKDRTAIQQRHDQLRDMVWIAEQLPGMMTKKDVTQGFLVPGNTSWLANGVPYFKNVLELSGVNYSEDQQLTVADEEELTSLASVDKYLRTHGFRGDLLGSQESIAYGNIDLKLFS YNARLGISDFHAFAGPVFLRFQHTQPRTLEDEGQDGGVPPAASMGDERLSVSIEDADSLAEMELITERRSVRNDMRAIAMRKIGSGPFKWSEMSPVEEGGGHEGHPDEWNFDKVSPKWAW* >PLBD2_acyPis Acyrthosiphon pisum (aphid) XM_001948827 MLSIRCILLSLLFVWALQCSATQKNQTLLAVKTDNNRITIQPKHYSVKDKEIIIGKGKFIDRINSTGWAYLEIRTSQKAKDEDQAYGAGYLEGTLTADLIYSYWFNTAKGYCTDRPNVCQQLK DYMTTNKNWIKSKLNESDPYWYQVGLYYKQLDGLYDGYMRGKSPSTPDLTWDDLYWLNALDDLGDLSIALYPSDISNRVLGSGSCSALIKLMPDNKDILVSHATWSGYETMLRIQKRYSLRFRKS KKSNKLIRGFDMSFSSFPGGIQSGDDFYLISSGLTTMETTIENYNDSLWSNVKPVGQVLEFVRAMVANRLADNPTDWANLFKLHNSGTYNNQWMILNYAAFQPGSPLPPRDVLHVLEQIPGHVMHD DFTGHLINRTYWASYNVPYFPFIFNVSGNYEMEQIYGSWFSYSETPRARIFARDHVKIHCDKCMLHLMRSNNYTRD PESRCDCSPPYSAENAISSRNDLNPANGTYPIRALGHRSHGATDVKVTSSQLFQQLQFKAIAGPTQGSNNSLGPFCWSKSDFNDKVSHLGHPDCFNFKPVLHQWSL* >PLBD2_triAdh Trichoplax adhaerens (trichoplax) XM_002107718 introns largely conserved 0 MAQCGKFLIYFSIFIITLATLCSCQSGSVIYKDGLYTFSKGINKRAASYGTFTDKIASSG 2 1 WTYLDVHTNPQDDDFITAYAAGYVEGILTAKY IYMHWKNTVGDYCKQKSIYCQKLKSFIMKNNQWMATQIKHRPHSIYWYH 0 0 INLTLIQQKGLRDGYHKAMPHKPIDEFSFL 2 1 LIELSGDLESLETALKDEDTHHVLGSGSCSAFIKVLPDNRDLYFAHDTWTGYQTMLRIYKYYELNFSMLPKTN 1 2 VTVPGTRISFSSYPGTILSGDDYYLIGSGL 0 0 ATMETTNGNSNEKLWKYVTPSSVLEWIRTIIANRLTSSGNDWVKIFSKYNSGT 2 1 YNNQ 00 WMILDYKLFAPKRPLNPNTLWVLEQIP 2 1 GKIESADVTNVLKKQGYWASYNVP 2 1 YFSSIFNMSGNQEQAKKYGNWFTHDKCPRALIFKRDQHKVNSMESLMKLMR 2 1 YNDFKHDPLSRCNCTPPYSAENAISARSDLNPADGKYNIGALGHRCHGGTDSK STNYTMFHSGLKSYAIAGPTHEQQPPFRWSTAKFNMTKPLGHPDLFNFTRQLVSWD* 0 >PLBD2_monBre Monosiga brevicollis (choanoflagellate) introns all novel 0 MWSCGAAAAAVVAVVVLASPATATVARFVEQTDVQTTYASVFYVESDDSYVVKTENHPWDGDFEKDE 0 0 AVRIKYTPGYLVAGWDQLHVKSNSAMDDATVAYAAGYGEAQLTAEMIYNYAYNNGYDTFTPNDKLADYLAKNQAFMAASIASNRSDANGYWYHVDLILRQLQGVCDGYNSSD FAKSFPLPCESMLAINLMGDMEDLSDALASSDEWYTEDRFFRATHCSALVKLVGGASSPSDIYISQDTWSSLNSMTRIMKRYDLNFLQ 2 1 AKGADDRIAGSSIVFSSYPGSLYSGDDFYLTSAGMAVIETTIGNSNPELYQYIVPDTVLEWIRNIMANRLASNSQTWYEVYRQFNSGT 1 2 YNNMNMILDYKQFKPQEALQDELLTIVEQIP GTVTKTDVTGYLRNMTYWGS 1 2 YNVAFDQNIRELSGANQAEQLYGPW 2 1 FSYWNTSRALIFAREQKNVSSLEDLKRLMRLNQFKTDPL 2 1 YRGWTNCTPAYTAENVIATRGDLNDP 0 0 NGIYSLSSFGLRNHVATDSKISTFSTYDSNNLNVWAIS 2 1 GPTNGPPPNQPVFNWSTSYYKDTRHRGMPEAFDFDWVNFNWPF* 0 >PLBD2_dicDis Dictyostelium discoideum (slime_mold) AAFI02000019 AF411829 introns both novel 0 MRVIRSLLLLTIAIIGSVLSQSSIDDGYTVFYSQPDNYYVKPGTFSNGVAQAIFSNEMMTTGWSFMSISSSEGLYPNDIIAAGAGYLEGYISQEMIYQNWMNMYNNEYHNVIGSD VENWIQENLQYLQTMIDSAPSNDLYWQNVETVLTQITYMQRGYNQSVIDNGVDASQSLGITEFFLMNMDGDMIDLGPALNLTNGKQVTSPATATSPKQAFKEFMRRTGHCSALIKMTDDLSDLFSGHTTW 2 1 SSYYEMVRMFKVYNLKYLFNGQPPASKVTMFSGYPGTLSSIDDFYLLDTKIVVIETTNGLMNNNLYHLITSESVLSWIRVIVANRLATGGESWCQTFSLYNSGTYNNQ 0 0 WIIVDYNKFIKGYGALDGTLYILEQVPDYVEYGDQTAILRTGYWPSFNIPFYENIYGLTGFNETYAQFGNWFSYQASPRSMIFKRDANNIHSLTQFQAMLRYNNWQNDPFSQGNAGN QISSRFDLVTADDPNNQYLDPDAFGGIDSKVVSADMVAALLVNAQSGPSHDNETPFTWNSQWNQKYTYAGQPTTWNFDWMTMSLQSMKPASPSSDSSSDSTTFN* 0