Bison: mitochondrial genomics
Introduction to bison and yak conservation genomics
(to be continued)
Phylogeny: bison and yak are sister species
Bison genomics is best considered within its phylogenetic context. This means first of all parallel consideration of its sister species (nearest living relative) the yak. Although not tropical, both species were dramatically affected by closing of the Darien gap in Panama at 2.5 million years and ensuing unstable climatic change. This led to Pleistocene ice ages: episodic glacial barriers isolating regional herds yet promoting repeated dispersion across Beringia as sea levels fell. Those events manifest today as deep bifurcations of the mitochondrial phylogenetic tree of both species.
However a broader phylogenetic perspective is also essential to provide the outgroup sequences that influence ancestral sequence reconstruction. Here the evolutionary history of cetartiodactyls has taken decades to sort out: the position of whales, once controversial, has been settled (sister, together with hippopotamus, of Ruminantia), as has the non-intuitive branching order of pigs and lamas (Camelidae are basal).
Within pecoran ruminants, difficulty arises not so much from conflicts between fossils morphology and molecular trees but rather rapid radiation of species (tree polytomy), only recently resolved (we hope and assume below) with the bovine SNP bead chip. This samples nuclear genes vastly better than homoplasy-prone microsatellites and sidesteps limitations of mitochondrial inheritance.
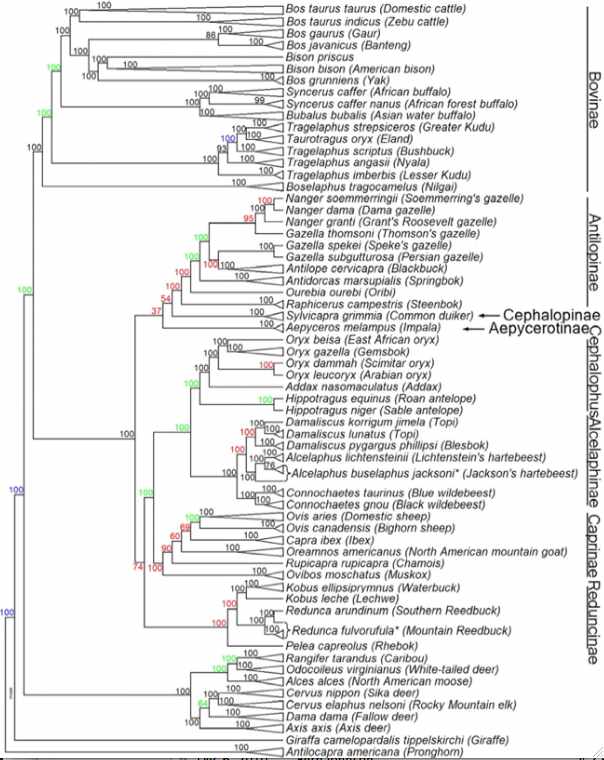
In the figure at left, JE Decker et al evaluated 52,356 sites across the nuclear genome not only of cows but throughout ruminants. The resulting tree (antelopes,(giraffes,(deer,((gazelles,sheep),bovinae)))) is critical to understanding the evolution of mitochondrial proteins and evaluating amino acid substitutions -- which are of grave concern for conservation of bottlenecked species such as bison and yak.
Notice that Linnaean taxonomy requires substantial revision according to the tree below -- genera such as Bos, Tragelaphus and Gazella are inconsistent with it. This could be remedied for bison by either placing them in Bos or putting yak, gaur and banteng in the genus Bison. Here the position of gaur and banteng has less bootstrap support than other nodes and has long been contentious. The position of kouprey and mithun (gayal), Bos sauveli and Bos frontalis, as not analyzed with the bead cheap.
There may not be any simplistic nomenclatural resolution because of male introgression as illustrated in european bison (wisent) and zebu cattle. The speciation process is far messier than suggested by bifurcating tree nodes. For example, subsequent to some measure of genomic divergence, wandering bulls from one population can join another or mixed herds of wild taurine cows form. While this does not affect mitochondrial lineages, it does result in periodic introgressions into the nuclear genomes. Since Holocene domestication, cattle have hybridized with aurochs, yak and bison, indicating full speciation barriers still do not exist. Polymorphic alleles represented in an ancestral population at various frequencies may sort out differently in descendant lineages, though this plays out quite differently for nuclear and mitochondrial genomes.
The data situation is otherwise very favorable with over 214 mammalian species having sequenced mitochondrial genomes, with high multiplicities for some individual species such as eland, cow, bison and yak. Individual genes such as CYTB may have extensive additional data from targeted studies. However all data, especially fragmentary older GenBank entries, must be carefully screened for errors and implausible sequence anomalies.
Acronm Species Common Mito CYTB Nuc PubMed bosSau Bos sauveli (kouprey) 0 5 0 15522811 16439342 17848372 bosFro Bos frontalis (mithun gayal) 0 16 0 20331596 20433524 18244904 17560527 bosGau Bos gaurus (gaur) 0 17 0 19436739 19777782 19367625 17986322 bosJav Bos javanicus (banteng) 2 39 0 18937038 18937038 17614913 16922247 12522420 bosTau Bos taurus (cattle) 168 500 1 19603063 19484124 19393053 19393048 19393045 20347826 bosPri Bos primigenius (auroch) 1 17 0 18199470 19456314 20346116 bosInd Bos indicus (zebu) 3 387 0 12648092 19436739 19770222 20597883 18467841 12399392 bosGru Bos grunniens (yak) 72 53 * 19917041 17257194 18439980 16942892 12137333 bisBis Bison bison (plains bison) 33 7 0 20870040 20637048 19414501 bisAth Bison athabascae (woods bison) 2 3 0 20808568 18191321 bisBon Bison bonasus (wisent eurobison) 4 9 0 14739241 19623210 17177698 15125253 14703870 bisPri Bison priscus (steppe bison) 0 0 0 15567864 20409351 20212118 18653730 18199470 bubBub Bubalus bubalis (water buffalo) 1 342 0 17459014 15621663 11212504 19140976 19462514 19207933 synCaf Syncerus caffer (cape buffalo) 0 10 0 10603253 9126673 9987926 17313588 17459014 14715223 traScr Tragelaphus scriptus (eland) 0 172 0 10222159 7723053 17520013 traSpp Tragelaphus others (7 spp eland) 0 7 0 10380679 bseTra Boselaphus tragocamelus (nilgai) 0 3 0 10603253 17158073
In the table, sequence availability counts do not include poor quality fragments or inadvertent hybrid data, eg the 13 Bos frontalis introgressions from Bos indicus are mislabeled at GenBank.
Yak nuclear genome sequencing is in progress at Beijing Genomics Institute. Other cetartiodactyl genomes in progress include Camelus bactrianus and Ovis aries with Camelus dromedarius and Pantholops hodgsonii completed but not released. Other relevant genomes said to be underway include Bubalus bubalis, Addax nasomaculatus, Muntiacus muntjak, Hippopotamus amphibius, and Balaena mysticetus. Cow, pig, sheep, and vicuna genomes have long been available for blast search.
These additional genomes would allow fossil nuclear numts to contribute to understanding of mitochondrial gene evolution, making the mitochondrial proteome of ancestral species such as Leptobos (last common ancestor to cattle, bison and yak) easy to work out. Note too that the mitochondrial genome, although not targeted, gets sequenced to very high multiplicity as a byproduct. To date, such projects have produced single mitochondrial genomes. This however is surely wrong in view of the prevalence of heteroplasmy: most species host a population of significantly different mitochondrial genomes. Thus these genome projects are a golden opportunity to characterize mitochondrial genome diversity within single species.
GenBank sequences are often retrieved blindly and run through extensive software pipelines to provide some conclusion. However it is imperative to manually curate accessions prior to analysis because a certain percentage of legacy entries are completely inappropriate. This ranges from attribution to the wrong species, gross and subtle sequence errors, reduced reliability at sequence termini, redundant entries, unpublishable submissions from third-world countries, mixups of mitochondrial and nuclear dna, lab dna contamination, text processing mishaps during the submission process, to outright data fraud. Below, bison and yak and their contextual species are considered individually.
- Both complete and fragmentary aurochs (Bos primigenius) accessions condense to two sequences sufficient to represent all GenBank aurochs data on 8 Dec 10 namely ACE76876 ADE05539 which differ as I4F T23A V372I (latter two changes are sporadic for ACE76876). Aurochsen became extinct in 1627 due to overhunting and the loss of habitat. Their mitochondrial genome still persists in a few Italian and Korean cows.
- The nine GenBank sequences for european bison (wisent) condense to a single representative sequence, for example ADF29596. Here it must be noted that ADQ12704 has a terrible sequencing error introducing ETTAEF for VNYGWI -- unfortunately this sequence has been used uncritically in published analyses. CAA75238 is also defective distally, a poor quality sequence from 2005 that was never published. Blast shows beyond any doubt that the known wisent sequences are not remotely affiliated with bison but instead are Bos taurus (not even aurochs Bos primigenius). It has long been suspected that wisent originated from a bison bull naturally crossed with a taurine cow. It follows wisent mitochondrial genomes will not be terribly informative for bison or yak.
- The extinct steppe bison, Bison priscus, has no protein sequences among its 298 GenBank entries, only control regions. Complete mitochondrial genomes from this species would be very informative -- evidently the dna is readily collected.
- The kouprey Bos sauveli has five CYTB sequences but only one full length, AAV51239. Two fragmentary entries are polymorphic relative to this at T248I, namely ABB88561 ABN73101. Here care must be taken as kouprey bull x banteng cow hybrids are known, causing confusion as to kouprey status as distinct wild cow species.
- Domestic cattle have a vast amount of sequence data, much breed-specific. The detail anomalies of inbred animals are not especially informative to wild bison or yak. Since however many of the sobserved cattle substitutions are radical chemical changes at highly conserved sites in a vital enzyme, the question arises as to how these animals survived to adulthood. The answer is probably heteroplasmy, with late onset, that is compensation via wildtype mitochondrial dna that persists in some mitochondria to some extent. Exercise intolerance -- a common outcome of human cytochrome b deficiency -- would hardly be noticed in a cow prior to the animal's arrival at a slaughterhouse. Two CYTB sequences have very high multiplicity, represented by AAM12814 AAW78524 at 208 and 71 copies. The latter differs at V356I I372V. The single-site polymorphisms shown below arise in AAV88174 BAC54760 AAZ16727 AAW83829 AAT80776 AAV88122 AAS93073 AAZ16896 AAT80776 AAZ95368 AAZ95339 AAS93061 AAM08329 AAZ95354 AAZ16545 AAZ95379 AAZ95338 AAZ95338 ABV70594 AAW78531 AAZ95334 AAZ95385 AAZ95378 ABV70763 ACQ73865 ACQ73761 AAZ95359 AAZ17091 BAA07016 AAV88161 ABV70555 AAZ95331 AAV88135 AAZ95405 AAZ95389 AAV88187 AAZ95386 AAZ16688 AAZ95339 AAQ06605 BAC20256 ACQ73813 AAW78527 respectively.
MTNIRKSHPLMKIVNNAFIDLPAPSNISSWWNFGSLLGICLILQILTGLFLAMHYTSDTTTAFSSVTHICRDVNYGWIIRYMHANGASMFFICLYMHVGRGLYYGSYTFLETWNIGVILLLTVMATAFMGYVLPWGQMSFWGATVITNLLSAIPYIGTNLVEWIWGGFSVDKATLTRFFAFHFILPFIIMAIAMVHLLFLHETGSNNPTGISSDVDKIPFHPYYTIKDILGALLLILALMLLVLFAPDLLGDPDNYTPANPLNTPPHIKPEWYFLFAYAILRSIPNKLGGVLALAFSILILALIPLLHTSKQRSMMFRPLSQCLFWALVADLLTLTWIGGQPVEHPYITIGQLASVLYFLLILVLMPTAGTIENKLLKW ................T..........................................................D...W...................................................L.........................................................K.........................................................N..............................................V.....S....................................................................S......... .................L...........................................................M............................................................................................N..................T...............................................................N...............................................T....................................................................AV....... ......................T......................................................V...................................................................................................................T....................................................................................................T.........................T...S....T.........................I...............V....... ..............................S..............................................V....................................................................................................................I.................................................................................................................................S....T.........................I....................... ................................................................................C.............................................................................................................................I.......................................................................................................................T.................................................... .........................................................................................V....................................................................................................................I....L.....................................................................................................................................S................................. ...................................................................................................G..................................................................................................................A....................................................................................................................................T............................... ......................................................................................................N...............................................................................................................A......................................................................................................................................F............................. ...............................................................................................................A......................................................................................................M............................................................................................................................................I....................... .....................................................................................................................L.................................................................................................N.........F......................................................................................................................................................... .......................................................................................................................................................................................................................................T...................................................................................................................................................
- The gayal (or mithun) Bos frontalis has 28 full length CYTB sequences. These fall into two very distinct groups, suggesting introgression of female mitochondria from another species. According to blastp, this species is Bos taurus or Bos indicus, a conclusion also reached for Yunnan gayal. Here ABO07421 most parsimoniously represents the first group should that be desired, with ACF17717 BAJ05325 identical, ABO07426 differing by a sporadic L376V, and ACN12147 differing by F296L and K375N. A derived subgroup has I356V and I372V and sporadic A291V, namely ABO07428 ABO07427 ABO07425 ABO07422 ACF17716 ABO07419.
- The second group of 16 near-identical gayal sequences can be represented by ABO07423. This set contains four sporadic mutations N3S D252N F276L I298L and two sites of shared polymorphism with the first group, T232A K375N. It differs consistently from the first group at 6 sites, I39V V215A A232T A302I A327T L357M and so bears much closer relationships -- given the strong conservation of this protein -- to Bos gaurus and secondarily Bos javanicus (2 and 4 differences respectively) than to Bos taurus or Bos indicus (6 differences at best). Only this second group is usefully included as an outgroup to yak and bison.
- Thus the first choice for Bos frontalis conservation genomics -- based solely on CYTB -- involves animals represented by the second group ABO07424 ACF17720 BAJ05320 ABO07423 ABO07420 ACF17718 ABS18292 AAV51237 BAJ05321 BAJ05322 ABS18291 with possible inclusion of ABO07418 ACF17719 for diversity but not sporadics BAJ05323 BAJ05324 ACM24710 unless other considerations warrant it. Based on skimpy GenBank entries, these animals are called Dulong cattle in China but mithun in Myanmar and Bhutan. This is apparently corroborated by a 2010 study utilizing 16S mitochondrial rRNA. Nuclear genes also are very important to consider.
- Bos gaurus has 17 entries including 3 where a nucleotide was submitted but not a translation (causing protein queries to miss them). After observing that the fragmentary sequences where not flawed are merely supportive, the set can be pruned to six. However two of these (ABF20228 ABF20227) are actually maternal Bos indicus/taurus sequences. The remaining four are practically identical to Bos javanicus/frontalis but differ from each other at 6 sporadic sites V39I A62V Y95H T108P L105P T190M N206I. This species will not prove useful to bison/yak comparative genomics but one sequence ADB80894 is retained below.
- The banteng Bos javanicus has an excellent set of complete sequences among its 35 entries for cytochrome b. After noting sporadic variation and checking for hybrids, a set of three sequences ABS18295 ABW82495 ABW82494 suffices to represent population diversity. Banteng do appear quite diverse, with several substantial variants supported by sequences from multiple individuals. Some clearly deleterious mutations are also evident, such as R80W in ADC53249. Sequences such as ABW82495 are peculiar in having 8 substitutions, suggesting a hybrid, yet with what is unclear: possibly a remote ancestor of Bos taurus or some extinct lineage not otherwise represented today. This sequence is supported by AAV51238 BAA11625 BAA07017 and so cannot be sequencing error; their disparate GenBank entries do not provide locational information.
- For the zebu, Bos indicus, 20 full length sequences are available (in addition to hundreds of fragments not considered further). These however are all identical with the exception of a sporadic variation T67I in ABS18290. Thus ABO07435 can serve to represent this species. It differs from the most abundant Bos taurus allele (208 entries) at only two distal positions I356V and V372I.
- Bison, yak and cattle have buffaloes as outgroup. Here Syncerus caffer (cape buffalo) has 10 CYTB sequences, only 2 of which are informative, AF036275 BAA11624. The latter differs at H3N T56S I295V.
- An extraordinary amount of data exists for water buffalo (Bubalus bubalis) -- some 165 CYTB sequences (after dropping defective entries ABO20788, ABO26586, BAJ05824 and discarding boundary variation of fragmentary sequences) of which 44 are essentially full length. However very little polymorphism occurs. In the first half of the molecule, 8 sites exhibit variation but only in unique individuals, making it impossible to distinguish sequencing error from authentic one-off events(which themselves could be non-heritable heteroplasmy. This is remarkably low (0.02%) in an alignment with 165 x 190 aa = 31,350 residues.
- The second half of Bubalus cytochrome b exhibits higher variation. Three individuals carry A191G, 28 have T246A, five are I365V and seven I372V, in addition to eight scattered sporadic variations. All the I372V individuals -- chinese water buffaloes -- are also T246A. The remaining 21 T246A animals apparently originated in China, Japan and Thailand but details remain unpublished. Non-sporadic variation in water buffalo is satisfactorily represented by GenBank accessions ACF17726 ABR08397.
- Syncerus is surprisingly diverged from Bubalus (12 positions): L102M T122A N159S I195V S246T I290V I293L L320F D331N M357T T371M. Only two of these positions are polymorphic in cape buffalo H3N and I295V; water buffalo are all 3N and 295V making those ancestral, with no indication of lineage sorting. This species is satisfactorily represented by AF036275 BAA11624.
- Can there be too much data? GenBank carries 172 CYTB sequences for Tragelaphus scriptus and its 30 subspecies (sylvaticus, uellensis, signatus, scriptus, simplex, sassae, roualeyni, punctatus, powelli, pictus, phaleratus, ornatus, meruensis, meridionalis, meneliki, massaicus, locorinae, knutsoni, johannae, heterochrous, haywoodi, fasciatus, dodingae, dianae, delameri, decula, dama, cottoni, bor, barkeri). However only two of these are full length, AF036277 AAD13501 (and differ at 7 sites) with the rest older and running from residue 138 to 232. Despite dropping poor quality sequences, considerable variation remains, both of sporadic and sub-clade type. To track this without sequences proliferating too much, a third quasi-sequence consisting of AF036277 substituted in silico with all major non-sporadic alleles -- which cannot represent sequencing error -- was made below, called CYTB_traScr3.
- Seven other species of Tragelaphus also have full length sequences available -- T. eurycerus, strepsiceros, imberbis, oryx, angasii, spekii, and derbianus. These sequences are moderately diverged from each other. They are fairly old in terms of sequencing technology used - 1999. Nonetheless, AAD51427 AAD51431 AAD13498 AAD13491 AAD42706 CAA10935 AAD13496 have been added to the sequence base below to represent this diversity. Tragelaphus is a large and important outgroup for bison/yak/cattle.
- Five of seven posted sequences for Boselaphus tragocamelus (nilgai) are poor quality fragments, illustrating a pitfall for blast searches. However the two full length sequences are in complete agreement. Here CAA10934 will be taken as reference sequence.
The goals here are to reduce the clutter from redundant sequences allowing an informative final alignment without discarding significant allele data or losing track of species multiplicities. This information can be retained within the alignment by a carefully designed fasta header. (Some web tools cut off the header at 10 characters but others allow any length.)
Interpreting bison CYTB variation
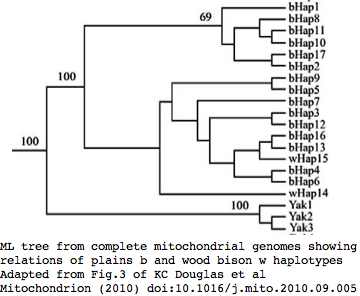
Bison mitochondrial genomes became well-represented at GenBank with the 1 Dec 10 release by the JN Derr group of 31 complete genomes (along with various cow-bison hybrids and cow breeds) from 6 herds including two woods bison (sometimes denoted Bison athabascae) from the presumably non-admixed Elk Island herd in Canada. Here cow-bison hybrids represent crossing of a bison male with a domestic cow (or rather a continuous line of female descent from such a cross) and so have strictly cow mitochondrial dna, not relevent to this section. The Derr group also posted a mitochondrial genome HQ223450 on 15 Nov 10 from european bison that -- like all of them to date -- was a taurine hybrid. The haplotype of all bison hybrids studied (from an unnamed private ranch in Montana conjectured the Flying D) cluster with cow haplotype cHap32 which may shed light on the historic cow lineage involved in late nineteenth century beefalo experiments.
Bison CYTB protein accessions: wood bison ADF48936 ADF48949 ADF48962 ADF48975 ADF48988 ADF49001 ADF49014 ADF49027 ADF49040 ADF49053 ADF49066 ADF49079 ADF49092 ADF49105 ADF49118 ADF49131 ADF49144 ADF49157 ADF49170 ADF49183 ADF49196 ADF49209 ADF49222 ADF49235 ADF49248 ADF49261 ADF49274 ADF49287 ADF49300 ADF49313 ADF49326 Earlier bison protein accessions: ABV70945 AAD51424 (ABV70945 complete genome: YP_002791041 derived from it; AAD51424 complete gene only) AAW28804 AAW28803 AAL85955 (fragmentary) ADM87433 (uninformative fragment) AAN28295 (taurine hybrid poor quality) Non-redundant protein set (with multiplicities): pick one from each row 18 98A: ADF49092 ADF49170 ADF49118 ADF49248 ADF49131 ADF49300 ADF48936 ADF48949 ADF48962 ADF49001 ADF49027 ADF49040 ADF49157 ADF49183 ADF49196 ADF49261 ADF49066 AAW28803 (frag) 1 98A V132D: AAD51424 1 98A Q322R: AAL85955 (frag) 13 V98: ADF49105 ADF49209 ADF49014 ADF48975 ADF49144 ADF49222 ADF49287 ADF49235 ADF49274 ADF49313 ADF48988 ADF49053 AAW28804 (frag) 1 V98 N3S: ADF49079 1 V98 I42T: ABV70945 1 V98 V123M: ADF49326
Thus for comparative genomics purposes, all available authentic bison cytochrome b data on 11 Dec 10 can be represented by just three sequences (one of them an artificial composite of all alleles). This facilitates comparison of polymorphism sites with yak and other species. The fasta headers are designed to display informatively after alignment. Apart from V98A, the other 5 variations are sporadic (observed in only one animal to date). They are analyzed in great detail below to determine which are deleterious mutations.
CYTB_bisBis_V98 wild type MTNLRKSHPLMKIVNNAFIDLPAPSNISSWWNFGSLLGMCLILQILTGLFLAMHYTSDTTTAFSSVAHICRDVNYGWIIRYMHANGASMFFICLYMHVGRGLYYGSYTFLETWNIGVILLLTVMATAFMGYVLPWGQMSF WGATVITNLLSAIPYIGTNLVEWIWGGFSVDKATLTRFFAFHFILPFIIMAIAMVHLLFLHETGSNNPTGISSDMDKIPFHPYYTIKDILGALLLILALMLLVLFTPDLLGDPDNYTPANPLNTPPHIKPEWYFLFAYAI LRSIPNKLGGVLALAFSILILALIPLLHTSKQRSMIFRPLSQCLFWTLVADLLTLTWIGGQPVEHPYIIIGQMASIMYFLLILVLMPTAGTIENKLLKW >CYTB_bisBis_98A major variant MTNLRKSHPLMKIVNNAFIDLPAPSNISSWWNFGSLLGMCLILQILTGLFLAMHYTSDTTTAFSSVAHICRDVNYGWIIRYMHANGASMFFICLYMHAGRGLYYGSYTFLETWNIGVILLLTVMATAFMGYVLPWGQMSF WGATVITNLLSAIPYIGTNLVEWIWGGFSVDKATLTRFFAFHFILPFIIMAIAMVHLLFLHETGSNNPTGISSDMDKIPFHPYYTIKDILGALLLILALMLLVLFTPDLLGDPDNYTPANPLNTPPHIKPEWYFLFAYAI LRSIPNKLGGVLALAFSILILALIPLLHTSKQRSMIFRPLSQCLFWTLVADLLTLTWIGGQPVEHPYIIIGQMASIMYFLLILVLMPTAGTIENKLLKW >CYTB_bisBis_all N3S I42T V98A V123M V132D Q322R all-allele composite MTSLRKSHPLMKIVNNAFIDLPAPSNISSWWNFGSLLGMCLTLQILTGLFLAMHYTSDTTTAFSSVAHICRDVNYGWIIRYMHANGASMFFICLYMHAGRGLYYGSYTFLETWNIGVILLLTMMATAFMGYDLPWGQMSF WGATVITNLLSAIPYIGTNLVEWIWGGFSVDKATLTRFFAFHFILPFIIMAIAMVHLLFLHETGSNNPTGISSDMDKIPFHPYYTIKDILGALLLILALMLLVLFTPDLLGDPDNYTPANPLNTPPHIKPEWYFLFAYAI LRSIPNKLGGVLALAFSILILALIPLLHTSKQRSMIFRPLSRCLFWTLVADLLTLTWIGGQPVEHPYIIIGQMASIMYFLLILVLMPTAGTIENKLLKW
N3S: Deleterious despite similarities of asperagine and serine. Based on 12603 CYTB sequences from 1637 species of mammals, this substitution has never gained traction.
4345 N 132 I 70 H 14 Y 9 K 5 S* 2 T Sus scrofa (pig) Platyrrhinus brachycephalus (bat) Naemorhedus caudatus (goral ungulate) Martes americana (marten carnivore)
A 17-fold identical group of sequences has A98, not including either wood bison nor matching outgroups yak and aurochs there nor other Bovinae: ADF49105 ADF49209 ADF49014 ADF49079 ADF49144 ADF49222 ADF49287 ADF49235 ADF49274 ADF49313 ADF48988 ADF49053 ADF49248. There are two sporadic variants in the V98 setting, namely L3S and V123M: ADF49079 ADF49326 (the latter the outlier wood bison wHap14). 123M may not sporadic if more animals related to Elk Island 14 can be found. The remaining group of 14 with consistent ancestral V98 are otherwise identical: ADF49092 ADF49170 ADF49118 ADF49248 ADF49131 ADF49300 ADF48936 ADF48949 ADF48962 ADF49001 ADF49027 ADF49040 ADF49157 ADF49183 ADF49196 ADF49261 ADF49066.
The single change V98A corresponds perfectly to the two major clades, with A98 shared by all individuals in the upper half of the tree ending in bHap2. In the overall mammalian context, A98 is a derived condition (synapomorpy) of the upper clade of bison. Note V98L is a domestic yak mutation described below. The vast majority of mammals are V98. Isoleucine is also common but methionine and alanine are quite rare among the 5000 sequences considered. The statistics: 4522 V, 430I, 34M, 11A, 1L, 1N.
The CYTB sequences retrieved from these genomic entries show haplotype notation. The 15 previously existing bison sequences at GenBank (some just fragments) are also provided. Older fragmentary sequences are demonstrably error-prone and will be used here only as support -- never as sole source -- of a polymorphism. Redundancy introduced via non-standard SwissProt (UniProt) entries also has to be manually removed -- the Swiss did no sequencing on their own, simply deriving protein sequences from existing GenBank entries. This leaves 5 older complete sequences for Bison bison and 4 fragments, 2 attributed to Bison bonasus and 1 fossil dna sequence from Bos primigenius to serve as outgroup (rather than an inbred domestic cow).
Here it is necessary to pick a terminology. This must accommodate NCBI taxonomy -- regardless of its correctness -- because otherwise blastp searches cannot be restricted by taxon. Note although bison are definitely sistered with yak to the exclusion of all other extant species, that creates problems because yak has been put in the genus Bos. Many relic wild cattle have no english language common name but rather that of a local language. Terminology table must show synonyms to allow PubMed and google searches -- especially important in a fast-moving field to locate preprints and conference proceedings. The table below does not attempt to implicitly resolve any scientific issue; it simply states preferred terminology at this site along with synonyms in common use.
Sequences are color clustered according to the phylogenetic tree above. bHap1 is not shown. Note the woods bison cannot be resolved from the plains bison even though the Elk Island woods bison are a relic herd that did not mix with 7,000 plains bison imported from the Flathead Reservation in Montana up to Canada's Wood Buffalo National Park in the 1920's. Clearly these animals are a mixture of the second major clade of bison with an earlier diverged lineage represented by wHap14 surviving (at least in mitochondrial dna) from the founder herd. This could represent allopatric separation during a glaciation epoch with subsequent reunification. However the prevalence of wHap14 needs to be established along with uniqueness of its nuclear dna.
NucAcc ProAcc PubMed ST ...... TYP MUT BP Change Isolate Haplo Source Herd GU946976 ADF48936 20870040 MT plains A98 V.98A GTA to GCA B790 bHap2 Montana private herd GU946977 ADF48949 20870040 MT plains A98 V.98A GTA to GCA B853 bHap2 Montana private herd GU946978 ADF48962 20870040 MT plains A98 V.98A GTA to GCA B854 bHap2 Montana private herd GU946981 ADF49001 20870040 MT plains A98 V.98A GTA to GCA B880 bHap2 Montana private herd GU946983 ADF49027 20870040 MT plains A98 V.98A GTA to GCA B925 bHap2 Montana private herd GU946984 ADF49040 20870040 MT plains A98 V.98A GTA to GCA B929 bHap2 Montana private herd GU946986 ADF49066 20870040 MT plains A98 V.98A GTA to GCA B959 bHap2 Montana private herd GU946993 ADF49157 20870040 MT plains A98 V.98A GTA to GCA B1029 bHap2 Montana private herd GU946995 ADF49183 20870040 MT plains A98 V.98A GTA to GCA B1050 bHap2 Montana private herd GU946996 ADF49196 20870040 MT plains A98 V.98A GTA to GCA B1051 bHap2 Montana private herd GU947001 ADF49261 20870040 NB plains A98 V.98A GTA to GCA BNBR1 bHap2 National Bison Refuge GU947004 ADF49300 20870040 YP plains A98 V.98A GTA to GCA BYNP1586 bHap17 Yellowstone Natl Park GU946990 ADF49118 20870040 MT plains A98 V.98A GTA to GCA B985 bHap10 Montana private herd GU946991 ADF49131 20870040 MT plains A98 V.98A GTA to GCA B1005 bHap10 Montana private herd GU947000 ADF49248 20870040 NB plains A98 V.98A GTA to GCA BFN5 bHap10 Fort Niobrara GU946994 ADF49170 20870040 MT plains A98 V.98A GTA to GCA B1031 bHap11 Montana private herd GU946988 ADF49092 20870040 MT plains A98 V.98A GTA to GCA B973 bHap8 Montana private herd AF036273 AAD51424 10603253 FR plains A98 V132D AT to TA ..... ..... Vincennes Zoo 1999 GU946979 ADF48975 20870040 MT plains V98 ..... .......... B855 bHap3 Montana private herd GU946992 ADF49144 20870040 MT plains V98 ..... .......... B1018 bHap3 Montana private herd GU946998 ADF49222 20870040 MT plains V98 ..... .......... B1191 bHap12 Montana private herd GU946980 ADF48988 20870040 MT plains V98 ..... .......... B877 bHap4 Montana private herd GU946985 ADF49053 20870040 MT plains V98 ..... .......... B935 bHap6 Montana private herd GU946989 ADF49105 20870040 MT plains V98 ..... .......... B979 bHap9 Montana private herd GU946997 ADF49209 20870040 MT plains V98 ..... .......... B1091 bHap9 Montana private herd GU946982 ADF49014 20870040 MT plains V98 ..... .......... B897 bHap5 Montana private herd GU947006 ADF49326 20870040 EI woodsB V98 V123M ATA to GTA wEI14 wHap14 Elk Island EU177871 ABV70945 18302915 IT plains V98 I.42T ATC to ACC ..... ..... unknown Italy GU946987 ADF49079 20870040 MT plains V98 N..3S AAC to AGC B961 bHap7 Montana private herd GU946999 ADF49235 20870040 MT plains V98 ..... .......... B1428 bHap13 Montana private herd GU947002 ADF49274 20870040 TX plains V98 ..... .......... BTSBH1001 bHap13 Texas Sate Bison Herd GU947003 ADF49287 20870040 TX plains V98 ..... .......... BTSBH1005 bHap16 Texas Sate Bison Herd GU947005 ADF49313 20870040 EI woodsB V98 ..... .......... wEI1 wHap15 Elk Island</font? >CYTB_bisBis.ADF49092 bHap8 plains bison b973 Montana A98 MTNLRKSHPLMKIVNNAFIDLPAPSNISSWWNFGSLLGMCLILQILTGLFLAMHYTSDTTTAFSSVAHICRDVNYGWIIRYMHANGASMFFICLYMHAGRGLYYGSYTFLETWNIGVILLLTVMATAFMGYVLPWGQMSF WGATVITNLLSAIPYIGTNLVEWIWGGFSVDKATLTRFFAFHFILPFIIMAIAMVHLLFLHETGSNNPTGISSDMDKIPFHPYYTIKDILGALLLILALMLLVLFTPDLLGDPDNYTPANPLNTPPHIKPEWYFLFAYAI LRSIPNKLGGVLALAFSILILALIPLLHTSKQRSMIFRPLSQCLFWTLVADLLTLTWIGGQPVEHPYIIIGQMASIMYFLLILVLMPTAGTIENKLLKW >CYTB_bisBis.ADF49170 bHap11 plains bison b1031 Montana A98 MTNLRKSHPLMKIVNNAFIDLPAPSNISSWWNFGSLLGMCLILQILTGLFLAMHYTSDTTTAFSSVAHICRDVNYGWIIRYMHANGASMFFICLYMHAGRGLYYGSYTFLETWNIGVILLLTVMATAFMGYVLPWGQMSF WGATVITNLLSAIPYIGTNLVEWIWGGFSVDKATLTRFFAFHFILPFIIMAIAMVHLLFLHETGSNNPTGISSDMDKIPFHPYYTIKDILGALLLILALMLLVLFTPDLLGDPDNYTPANPLNTPPHIKPEWYFLFAYAI LRSIPNKLGGVLALAFSILILALIPLLHTSKQRSMIFRPLSQCLFWTLVADLLTLTWIGGQPVEHPYIIIGQMASIMYFLLILVLMPTAGTIENKLLKW >CYTB_bisBis.ADF49118 bHap10 plains bison b985 Montana A98 MTNLRKSHPLMKIVNNAFIDLPAPSNISSWWNFGSLLGMCLILQILTGLFLAMHYTSDTTTAFSSVAHICRDVNYGWIIRYMHANGASMFFICLYMHAGRGLYYGSYTFLETWNIGVILLLTVMATAFMGYVLPWGQMSF WGATVITNLLSAIPYIGTNLVEWIWGGFSVDKATLTRFFAFHFILPFIIMAIAMVHLLFLHETGSNNPTGISSDMDKIPFHPYYTIKDILGALLLILALMLLVLFTPDLLGDPDNYTPANPLNTPPHIKPEWYFLFAYAI LRSIPNKLGGVLALAFSILILALIPLLHTSKQRSMIFRPLSQCLFWTLVADLLTLTWIGGQPVEHPYIIIGQMASIMYFLLILVLMPTAGTIENKLLKW >CYTB_bisBis.ADF49248 bHap10 plains bison bFN5 Niobrara A98 MTNLRKSHPLMKIVNNAFIDLPAPSNISSWWNFGSLLGMCLILQILTGLFLAMHYTSDTTTAFSSVAHICRDVNYGWIIRYMHANGASMFFICLYMHAGRGLYYGSYTFLETWNIGVILLLTVMATAFMGYVLPWGQMSF WGATVITNLLSAIPYIGTNLVEWIWGGFSVDKATLTRFFAFHFILPFIIMAIAMVHLLFLHETGSNNPTGISSDMDKIPFHPYYTIKDILGALLLILALMLLVLFTPDLLGDPDNYTPANPLNTPPHIKPEWYFLFAYAI LRSIPNKLGGVLALAFSILILALIPLLHTSKQRSMIFRPLSQCLFWTLVADLLTLTWIGGQPVEHPYIIIGQMASIMYFLLILVLMPTAGTIENKLLKW >CYTB_bisBis.ADF49131 bHap10 plains bison b1005 Montana A98 MTNLRKSHPLMKIVNNAFIDLPAPSNISSWWNFGSLLGMCLILQILTGLFLAMHYTSDTTTAFSSVAHICRDVNYGWIIRYMHANGASMFFICLYMHAGRGLYYGSYTFLETWNIGVILLLTVMATAFMGYVLPWGQMSF WGATVITNLLSAIPYIGTNLVEWIWGGFSVDKATLTRFFAFHFILPFIIMAIAMVHLLFLHETGSNNPTGISSDMDKIPFHPYYTIKDILGALLLILALMLLVLFTPDLLGDPDNYTPANPLNTPPHIKPEWYFLFAYAI LRSIPNKLGGVLALAFSILILALIPLLHTSKQRSMIFRPLSQCLFWTLVADLLTLTWIGGQPVEHPYIIIGQMASIMYFLLILVLMPTAGTIENKLLKW >CYTB_bisBis.ADF49300 bHap17 plains bison bYNP1586 Yellowstone A98 MTNLRKSHPLMKIVNNAFIDLPAPSNISSWWNFGSLLGMCLILQILTGLFLAMHYTSDTTTAFSSVAHICRDVNYGWIIRYMHANGASMFFICLYMHAGRGLYYGSYTFLETWNIGVILLLTVMATAFMGYVLPWGQMSF WGATVITNLLSAIPYIGTNLVEWIWGGFSVDKATLTRFFAFHFILPFIIMAIAMVHLLFLHETGSNNPTGISSDMDKIPFHPYYTIKDILGALLLILALMLLVLFTPDLLGDPDNYTPANPLNTPPHIKPEWYFLFAYAI LRSIPNKLGGVLALAFSILILALIPLLHTSKQRSMIFRPLSQCLFWTLVADLLTLTWIGGQPVEHPYIIIGQMASIMYFLLILVLMPTAGTIENKLLKW >CYTB_bisBis.ADF48936 bHap2 plains bison b790 Montana A98 MTNLRKSHPLMKIVNNAFIDLPAPSNISSWWNFGSLLGMCLILQILTGLFLAMHYTSDTTTAFSSVAHICRDVNYGWIIRYMHANGASMFFICLYMHAGRGLYYGSYTFLETWNIGVILLLTVMATAFMGYVLPWGQMSF WGATVITNLLSAIPYIGTNLVEWIWGGFSVDKATLTRFFAFHFILPFIIMAIAMVHLLFLHETGSNNPTGISSDMDKIPFHPYYTIKDILGALLLILALMLLVLFTPDLLGDPDNYTPANPLNTPPHIKPEWYFLFAYAI LRSIPNKLGGVLALAFSILILALIPLLHTSKQRSMIFRPLSQCLFWTLVADLLTLTWIGGQPVEHPYIIIGQMASIMYFLLILVLMPTAGTIENKLLKW >CYTB_bisBis.ADF48949 bHap2 plains bison b853 Montana A98 MTNLRKSHPLMKIVNNAFIDLPAPSNISSWWNFGSLLGMCLILQILTGLFLAMHYTSDTTTAFSSVAHICRDVNYGWIIRYMHANGASMFFICLYMHAGRGLYYGSYTFLETWNIGVILLLTVMATAFMGYVLPWGQMSF WGATVITNLLSAIPYIGTNLVEWIWGGFSVDKATLTRFFAFHFILPFIIMAIAMVHLLFLHETGSNNPTGISSDMDKIPFHPYYTIKDILGALLLILALMLLVLFTPDLLGDPDNYTPANPLNTPPHIKPEWYFLFAYAI LRSIPNKLGGVLALAFSILILALIPLLHTSKQRSMIFRPLSQCLFWTLVADLLTLTWIGGQPVEHPYIIIGQMASIMYFLLILVLMPTAGTIENKLLKW >CYTB_bisBis.ADF48962 bHap2 plains bison b854 Montana A98 MTNLRKSHPLMKIVNNAFIDLPAPSNISSWWNFGSLLGMCLILQILTGLFLAMHYTSDTTTAFSSVAHICRDVNYGWIIRYMHANGASMFFICLYMHAGRGLYYGSYTFLETWNIGVILLLTVMATAFMGYVLPWGQMSF WGATVITNLLSAIPYIGTNLVEWIWGGFSVDKATLTRFFAFHFILPFIIMAIAMVHLLFLHETGSNNPTGISSDMDKIPFHPYYTIKDILGALLLILALMLLVLFTPDLLGDPDNYTPANPLNTPPHIKPEWYFLFAYAI LRSIPNKLGGVLALAFSILILALIPLLHTSKQRSMIFRPLSQCLFWTLVADLLTLTWIGGQPVEHPYIIIGQMASIMYFLLILVLMPTAGTIENKLLKW >CYTB_bisBis.ADF49001 bHap2 plains bison b880 Montana A98 MTNLRKSHPLMKIVNNAFIDLPAPSNISSWWNFGSLLGMCLILQILTGLFLAMHYTSDTTTAFSSVAHICRDVNYGWIIRYMHANGASMFFICLYMHAGRGLYYGSYTFLETWNIGVILLLTVMATAFMGYVLPWGQMSF WGATVITNLLSAIPYIGTNLVEWIWGGFSVDKATLTRFFAFHFILPFIIMAIAMVHLLFLHETGSNNPTGISSDMDKIPFHPYYTIKDILGALLLILALMLLVLFTPDLLGDPDNYTPANPLNTPPHIKPEWYFLFAYAI LRSIPNKLGGVLALAFSILILALIPLLHTSKQRSMIFRPLSQCLFWTLVADLLTLTWIGGQPVEHPYIIIGQMASIMYFLLILVLMPTAGTIENKLLKW >CYTB_bisBis.ADF49027 bHap2 plains bison b925 Montana A98 MTNLRKSHPLMKIVNNAFIDLPAPSNISSWWNFGSLLGMCLILQILTGLFLAMHYTSDTTTAFSSVAHICRDVNYGWIIRYMHANGASMFFICLYMHAGRGLYYGSYTFLETWNIGVILLLTVMATAFMGYVLPWGQMSF WGATVITNLLSAIPYIGTNLVEWIWGGFSVDKATLTRFFAFHFILPFIIMAIAMVHLLFLHETGSNNPTGISSDMDKIPFHPYYTIKDILGALLLILALMLLVLFTPDLLGDPDNYTPANPLNTPPHIKPEWYFLFAYAI LRSIPNKLGGVLALAFSILILALIPLLHTSKQRSMIFRPLSQCLFWTLVADLLTLTWIGGQPVEHPYIIIGQMASIMYFLLILVLMPTAGTIENKLLKW >CYTB_bisBis.ADF49040 bHap2 plains bison b929 Montana A98 MTNLRKSHPLMKIVNNAFIDLPAPSNISSWWNFGSLLGMCLILQILTGLFLAMHYTSDTTTAFSSVAHICRDVNYGWIIRYMHANGASMFFICLYMHAGRGLYYGSYTFLETWNIGVILLLTVMATAFMGYVLPWGQMSF WGATVITNLLSAIPYIGTNLVEWIWGGFSVDKATLTRFFAFHFILPFIIMAIAMVHLLFLHETGSNNPTGISSDMDKIPFHPYYTIKDILGALLLILALMLLVLFTPDLLGDPDNYTPANPLNTPPHIKPEWYFLFAYAI LRSIPNKLGGVLALAFSILILALIPLLHTSKQRSMIFRPLSQCLFWTLVADLLTLTWIGGQPVEHPYIIIGQMASIMYFLLILVLMPTAGTIENKLLKW >CYTB_bisBis.ADF49157 bHap2 plains bison b1029 Montana A98 MTNLRKSHPLMKIVNNAFIDLPAPSNISSWWNFGSLLGMCLILQILTGLFLAMHYTSDTTTAFSSVAHICRDVNYGWIIRYMHANGASMFFICLYMHAGRGLYYGSYTFLETWNIGVILLLTVMATAFMGYVLPWGQMSF WGATVITNLLSAIPYIGTNLVEWIWGGFSVDKATLTRFFAFHFILPFIIMAIAMVHLLFLHETGSNNPTGISSDMDKIPFHPYYTIKDILGALLLILALMLLVLFTPDLLGDPDNYTPANPLNTPPHIKPEWYFLFAYAI LRSIPNKLGGVLALAFSILILALIPLLHTSKQRSMIFRPLSQCLFWTLVADLLTLTWIGGQPVEHPYIIIGQMASIMYFLLILVLMPTAGTIENKLLKW >CYTB_bisBis.ADF49183 bHap2 plains bison b1050 Montana A98 MTNLRKSHPLMKIVNNAFIDLPAPSNISSWWNFGSLLGMCLILQILTGLFLAMHYTSDTTTAFSSVAHICRDVNYGWIIRYMHANGASMFFICLYMHAGRGLYYGSYTFLETWNIGVILLLTVMATAFMGYVLPWGQMSF WGATVITNLLSAIPYIGTNLVEWIWGGFSVDKATLTRFFAFHFILPFIIMAIAMVHLLFLHETGSNNPTGISSDMDKIPFHPYYTIKDILGALLLILALMLLVLFTPDLLGDPDNYTPANPLNTPPHIKPEWYFLFAYAI LRSIPNKLGGVLALAFSILILALIPLLHTSKQRSMIFRPLSQCLFWTLVADLLTLTWIGGQPVEHPYIIIGQMASIMYFLLILVLMPTAGTIENKLLKW >CYTB_bisBis.ADF49196 bHap2 plains bison b1051 Montana A98 MTNLRKSHPLMKIVNNAFIDLPAPSNISSWWNFGSLLGMCLILQILTGLFLAMHYTSDTTTAFSSVAHICRDVNYGWIIRYMHANGASMFFICLYMHAGRGLYYGSYTFLETWNIGVILLLTVMATAFMGYVLPWGQMSF WGATVITNLLSAIPYIGTNLVEWIWGGFSVDKATLTRFFAFHFILPFIIMAIAMVHLLFLHETGSNNPTGISSDMDKIPFHPYYTIKDILGALLLILALMLLVLFTPDLLGDPDNYTPANPLNTPPHIKPEWYFLFAYAI LRSIPNKLGGVLALAFSILILALIPLLHTSKQRSMIFRPLSQCLFWTLVADLLTLTWIGGQPVEHPYIIIGQMASIMYFLLILVLMPTAGTIENKLLKW >CYTB_bisBis.ADF49261 bHap2 plains bison bNBR1 National Bison Range A98 MTNLRKSHPLMKIVNNAFIDLPAPSNISSWWNFGSLLGMCLILQILTGLFLAMHYTSDTTTAFSSVAHICRDVNYGWIIRYMHANGASMFFICLYMHAGRGLYYGSYTFLETWNIGVILLLTVMATAFMGYVLPWGQMSF WGATVITNLLSAIPYIGTNLVEWIWGGFSVDKATLTRFFAFHFILPFIIMAIAMVHLLFLHETGSNNPTGISSDMDKIPFHPYYTIKDILGALLLILALMLLVLFTPDLLGDPDNYTPANPLNTPPHIKPEWYFLFAYAI LRSIPNKLGGVLALAFSILILALIPLLHTSKQRSMIFRPLSQCLFWTLVADLLTLTWIGGQPVEHPYIIIGQMASIMYFLLILVLMPTAGTIENKLLKW >CYTB_bisBis.ADF49066 bHap2 plains bison b959 Montana A98 MTNLRKSHPLMKIVNNAFIDLPAPSNISSWWNFGSLLGMCLILQILTGLFLAMHYTSDTTTAFSSVAHICRDVNYGWIIRYMHANGASMFFICLYMHAGRGLYYGSYTFLETWNIGVILLLTVMATAFMGYVLPWGQMSF WGATVITNLLSAIPYIGTNLVEWIWGGFSVDKATLTRFFAFHFILPFIIMAIAMVHLLFLHETGSNNPTGISSDMDKIPFHPYYTIKDILGALLLILALMLLVLFTPDLLGDPDNYTPANPLNTPPHIKPEWYFLFAYAI LRSIPNKLGGVLALAFSILILALIPLLHTSKQRSMIFRPLSQCLFWTLVADLLTLTWIGGQPVEHPYIIIGQMASIMYFLLILVLMPTAGTIENKLLKW >CYTB_bisBis.ADF49105 bHap9 plains bison b979 Montana V98 MTNLRKSHPLMKIVNNAFIDLPAPSNISSWWNFGSLLGMCLILQILTGLFLAMHYTSDTTTAFSSVAHICRDVNYGWIIRYMHANGASMFFICLYMHVGRGLYYGSYTFLETWNIGVILLLTVMATAFMGYVLPWGQMSF WGATVITNLLSAIPYIGTNLVEWIWGGFSVDKATLTRFFAFHFILPFIIMAIAMVHLLFLHETGSNNPTGISSDMDKIPFHPYYTIKDILGALLLILALMLLVLFTPDLLGDPDNYTPANPLNTPPHIKPEWYFLFAYAI LRSIPNKLGGVLALAFSILILALIPLLHTSKQRSMIFRPLSQCLFWTLVADLLTLTWIGGQPVEHPYIIIGQMASIMYFLLILVLMPTAGTIENKLLKW >CYTB_bisBis.ADF49209 bHap9 plains bison b1091 Montana V98 MTNLRKSHPLMKIVNNAFIDLPAPSNISSWWNFGSLLGMCLILQILTGLFLAMHYTSDTTTAFSSVAHICRDVNYGWIIRYMHANGASMFFICLYMHVGRGLYYGSYTFLETWNIGVILLLTVMATAFMGYVLPWGQMSF WGATVITNLLSAIPYIGTNLVEWIWGGFSVDKATLTRFFAFHFILPFIIMAIAMVHLLFLHETGSNNPTGISSDMDKIPFHPYYTIKDILGALLLILALMLLVLFTPDLLGDPDNYTPANPLNTPPHIKPEWYFLFAYAI LRSIPNKLGGVLALAFSILILALIPLLHTSKQRSMIFRPLSQCLFWTLVADLLTLTWIGGQPVEHPYIIIGQMASIMYFLLILVLMPTAGTIENKLLKW >CYTB_bisBis.ADF49014 bHap5 plains bison b897 Montana V98 MTNLRKSHPLMKIVNNAFIDLPAPSNISSWWNFGSLLGMCLILQILTGLFLAMHYTSDTTTAFSSVAHICRDVNYGWIIRYMHANGASMFFICLYMHVGRGLYYGSYTFLETWNIGVILLLTVMATAFMGYVLPWGQMSF WGATVITNLLSAIPYIGTNLVEWIWGGFSVDKATLTRFFAFHFILPFIIMAIAMVHLLFLHETGSNNPTGISSDMDKIPFHPYYTIKDILGALLLILALMLLVLFTPDLLGDPDNYTPANPLNTPPHIKPEWYFLFAYAI LRSIPNKLGGVLALAFSILILALIPLLHTSKQRSMIFRPLSQCLFWTLVADLLTLTWIGGQPVEHPYIIIGQMASIMYFLLILVLMPTAGTIENKLLKW >CYTB_bisBis.ADF49079 bHap7 plains bison b961 Montana N3S V98 MTSLRKSHPLMKIVNNAFIDLPAPSNISSWWNFGSLLGMCLILQILTGLFLAMHYTSDTTTAFSSVAHICRDVNYGWIIRYMHANGASMFFICLYMHVGRGLYYGSYTFLETWNIGVILLLTVMATAFMGYVLPWGQMSF WGATVITNLLSAIPYIGTNLVEWIWGGFSVDKATLTRFFAFHFILPFIIMAIAMVHLLFLHETGSNNPTGISSDMDKIPFHPYYTIKDILGALLLILALMLLVLFTPDLLGDPDNYTPANPLNTPPHIKPEWYFLFAYAI LRSIPNKLGGVLALAFSILILALIPLLHTSKQRSMIFRPLSQCLFWTLVADLLTLTWIGGQPVEHPYIIIGQMASIMYFLLILVLMPTAGTIENKLLKW >CYTB_bisBis.ADF48975 bHap3 plains bison b855 Montana V98 MTNLRKSHPLMKIVNNAFIDLPAPSNISSWWNFGSLLGMCLILQILTGLFLAMHYTSDTTTAFSSVAHICRDVNYGWIIRYMHANGASMFFICLYMHVGRGLYYGSYTFLETWNIGVILLLTVMATAFMGYVLPWGQMSF WGATVITNLLSAIPYIGTNLVEWIWGGFSVDKATLTRFFAFHFILPFIIMAIAMVHLLFLHETGSNNPTGISSDMDKIPFHPYYTIKDILGALLLILALMLLVLFTPDLLGDPDNYTPANPLNTPPHIKPEWYFLFAYAI LRSIPNKLGGVLALAFSILILALIPLLHTSKQRSMIFRPLSQCLFWTLVADLLTLTWIGGQPVEHPYIIIGQMASIMYFLLILVLMPTAGTIENKLLKW >CYTB_bisBis.ADF49144 bHap3 plains bison b1018 Montana V98 MTNLRKSHPLMKIVNNAFIDLPAPSNISSWWNFGSLLGMCLILQILTGLFLAMHYTSDTTTAFSSVAHICRDVNYGWIIRYMHANGASMFFICLYMHVGRGLYYGSYTFLETWNIGVILLLTVMATAFMGYVLPWGQMSF WGATVITNLLSAIPYIGTNLVEWIWGGFSVDKATLTRFFAFHFILPFIIMAIAMVHLLFLHETGSNNPTGISSDMDKIPFHPYYTIKDILGALLLILALMLLVLFTPDLLGDPDNYTPANPLNTPPHIKPEWYFLFAYAI LRSIPNKLGGVLALAFSILILALIPLLHTSKQRSMIFRPLSQCLFWTLVADLLTLTWIGGQPVEHPYIIIGQMASIMYFLLILVLMPTAGTIENKLLKW >CYTB_bisBis.ADF49222 bHap12 plains bison b1191 Montana V98 MTNLRKSHPLMKIVNNAFIDLPAPSNISSWWNFGSLLGMCLILQILTGLFLAMHYTSDTTTAFSSVAHICRDVNYGWIIRYMHANGASMFFICLYMHVGRGLYYGSYTFLETWNIGVILLLTVMATAFMGYVLPWGQMSF WGATVITNLLSAIPYIGTNLVEWIWGGFSVDKATLTRFFAFHFILPFIIMAIAMVHLLFLHETGSNNPTGISSDMDKIPFHPYYTIKDILGALLLILALMLLVLFTPDLLGDPDNYTPANPLNTPPHIKPEWYFLFAYAI LRSIPNKLGGVLALAFSILILALIPLLHTSKQRSMIFRPLSQCLFWTLVADLLTLTWIGGQPVEHPYIIIGQMASIMYFLLILVLMPTAGTIENKLLKW >CYTB_bisBis.ADF49287 bHap16 plains bison bTSBH1005 Texas State Bison Herd V98 MTNLRKSHPLMKIVNNAFIDLPAPSNISSWWNFGSLLGMCLILQILTGLFLAMHYTSDTTTAFSSVAHICRDVNYGWIIRYMHANGASMFFICLYMHVGRGLYYGSYTFLETWNIGVILLLTVMATAFMGYVLPWGQMSF WGATVITNLLSAIPYIGTNLVEWIWGGFSVDKATLTRFFAFHFILPFIIMAIAMVHLLFLHETGSNNPTGISSDMDKIPFHPYYTIKDILGALLLILALMLLVLFTPDLLGDPDNYTPANPLNTPPHIKPEWYFLFAYAI LRSIPNKLGGVLALAFSILILALIPLLHTSKQRSMIFRPLSQCLFWTLVADLLTLTWIGGQPVEHPYIIIGQMASIMYFLLILVLMPTAGTIENKLLKW >CYTB_bisBis.ADF49235 bHap13 plains bison b1428 Montana V98 MTNLRKSHPLMKIVNNAFIDLPAPSNISSWWNFGSLLGMCLILQILTGLFLAMHYTSDTTTAFSSVAHICRDVNYGWIIRYMHANGASMFFICLYMHVGRGLYYGSYTFLETWNIGVILLLTVMATAFMGYVLPWGQMSF WGATVITNLLSAIPYIGTNLVEWIWGGFSVDKATLTRFFAFHFILPFIIMAIAMVHLLFLHETGSNNPTGISSDMDKIPFHPYYTIKDILGALLLILALMLLVLFTPDLLGDPDNYTPANPLNTPPHIKPEWYFLFAYAI LRSIPNKLGGVLALAFSILILALIPLLHTSKQRSMIFRPLSQCLFWTLVADLLTLTWIGGQPVEHPYIIIGQMASIMYFLLILVLMPTAGTIENKLLKW >CYTB_bisBis.ADF49274 bHap13 plains bison bTSBH1001 Texas State Bison Herd V98 MTNLRKSHPLMKIVNNAFIDLPAPSNISSWWNFGSLLGMCLILQILTGLFLAMHYTSDTTTAFSSVAHICRDVNYGWIIRYMHANGASMFFICLYMHVGRGLYYGSYTFLETWNIGVILLLTVMATAFMGYVLPWGQMSF WGATVITNLLSAIPYIGTNLVEWIWGGFSVDKATLTRFFAFHFILPFIIMAIAMVHLLFLHETGSNNPTGISSDMDKIPFHPYYTIKDILGALLLILALMLLVLFTPDLLGDPDNYTPANPLNTPPHIKPEWYFLFAYAI LRSIPNKLGGVLALAFSILILALIPLLHTSKQRSMIFRPLSQCLFWTLVADLLTLTWIGGQPVEHPYIIIGQMASIMYFLLILVLMPTAGTIENKLLKW >CYTB_bisAth.ADF49313 wHap15 woods bison wEI1 Elk Island V98 MTNLRKSHPLMKIVNNAFIDLPAPSNISSWWNFGSLLGMCLILQILTGLFLAMHYTSDTTTAFSSVAHICRDVNYGWIIRYMHANGASMFFICLYMHVGRGLYYGSYTFLETWNIGVILLLTVMATAFMGYVLPWGQMSF WGATVITNLLSAIPYIGTNLVEWIWGGFSVDKATLTRFFAFHFILPFIIMAIAMVHLLFLHETGSNNPTGISSDMDKIPFHPYYTIKDILGALLLILALMLLVLFTPDLLGDPDNYTPANPLNTPPHIKPEWYFLFAYAI LRSIPNKLGGVLALAFSILILALIPLLHTSKQRSMIFRPLSQCLFWTLVADLLTLTWIGGQPVEHPYIIIGQMASIMYFLLILVLMPTAGTIENKLLKW >CYTB_bisBis.ADF48988 bHap4 plains bison b877 Montana V98 MTNLRKSHPLMKIVNNAFIDLPAPSNISSWWNFGSLLGMCLILQILTGLFLAMHYTSDTTTAFSSVAHICRDVNYGWIIRYMHANGASMFFICLYMHVGRGLYYGSYTFLETWNIGVILLLTVMATAFMGYVLPWGQMSF WGATVITNLLSAIPYIGTNLVEWIWGGFSVDKATLTRFFAFHFILPFIIMAIAMVHLLFLHETGSNNPTGISSDMDKIPFHPYYTIKDILGALLLILALMLLVLFTPDLLGDPDNYTPANPLNTPPHIKPEWYFLFAYAI LRSIPNKLGGVLALAFSILILALIPLLHTSKQRSMIFRPLSQCLFWTLVADLLTLTWIGGQPVEHPYIIIGQMASIMYFLLILVLMPTAGTIENKLLKW >CYTB_bisBis.ADF49053 bHap6 plains bison b935 Montana V98 MTNLRKSHPLMKIVNNAFIDLPAPSNISSWWNFGSLLGMCLILQILTGLFLAMHYTSDTTTAFSSVAHICRDVNYGWIIRYMHANGASMFFICLYMHVGRGLYYGSYTFLETWNIGVILLLTVMATAFMGYVLPWGQMSF WGATVITNLLSAIPYIGTNLVEWIWGGFSVDKATLTRFFAFHFILPFIIMAIAMVHLLFLHETGSNNPTGISSDMDKIPFHPYYTIKDILGALLLILALMLLVLFTPDLLGDPDNYTPANPLNTPPHIKPEWYFLFAYAI LRSIPNKLGGVLALAFSILILALIPLLHTSKQRSMIFRPLSQCLFWTLVADLLTLTWIGGQPVEHPYIIIGQMASIMYFLLILVLMPTAGTIENKLLKW >CYTB_bisAth.ADF49326 wHap14 woods bison wEI14 Elk Island V98 V123M MTNLRKSHPLMKIVNNAFIDLPAPSNISSWWNFGSLLGMCLILQILTGLFLAMHYTSDTTTAFSSVAHICRDVNYGWIIRYMHANGASMFFICLYMHVGRGLYYGSYTFLETWNIGVILLLTMMATAFMGYVLPWGQMSF WGATVITNLLSAIPYIGTNLVEWIWGGFSVDKATLTRFFAFHFILPFIIMAIAMVHLLFLHETGSNNPTGISSDMDKIPFHPYYTIKDILGALLLILALMLLVLFTPDLLGDPDNYTPANPLNTPPHIKPEWYFLFAYAI LRSIPNKLGGVLALAFSILILALIPLLHTSKQRSMIFRPLSQCLFWTLVADLLTLTWIGGQPVEHPYIIIGQMASIMYFLLILVLMPTAGTIENKLLKW >CYTB_bisBis_V98_I42T ABV70945 V98 I42T Bison bison MTNLRKSHPLMKIVNNAFIDLPAPSNISSWWNFGSLLGMCLTLQILTGLFLAMHYTSDTTTAFSSVAHICRDVNYGWIIRYMHANGASMFFICLYMHVGRGLYYGSYTFLETWNIGVILLLTVMATAFMGYVLPWGQMSF WGATVITNLLSAIPYIGTNLVEWIWGGFSVDKATLTRFFAFHFILPFIIMAIAMVHLLFLHETGSNNPTGISSDMDKIPFHPYYTIKDILGALLLILALMLLVLFTPDLLGDPDNYTPANPLNTPPHIKPEWYFLFAYAI LRSIPNKLGGVLALAFSILILALIPLLHTSKQRSMIFRPLSQCLFWTLVADLLTLTWIGGQPVEHPYIIIGQMASIMYFLLILVLMPTAGTIENKLLKW >CYTB_bisBis_98A_V132D AAD51424 Bison bison MTNLRKSHPLMKIVNNAFIDLPAPSNISSWWNFGSLLGMCLILXILTGLFLAMHYTSDTTTAFSSVAHICRDVNYGWIIRYMHANGASMFFICLYMHAGRGLYYGSYTFLETWNIGVILLLTVMATAFMGYDLPWGQMSF WGATVITNLLSAIPYIGTNLVEWIWGGFSVDKATLTRFFAFHFILPFIIMAIAMVHLLFLHETGSNNPTGISSDMDKIPFHPYYTIKDILGALLLILALMLLVLFTPDLLGDPDNYTPANPLNTPPHIKPEWYFLFAYAI LRSIPNKLGGVLALAFSILILALIPLLHTSKQRSMIFRPLSQCLFWTLVADLLTLTWIGGQPVEHPYIIIGQMASIMYFLLILVLMPTAGTIENKLLKW >CYTB_bisBis_V98 AAW28804 Bison bison NFGSLLGMCLILQILTGLFLAMHYTSDTTTAFSSVAHICRDVNYGWIIRYMHANGASMFFICLYMHVGRGLYYGSYTFLETWNIGVILLLTVMATAFMGYVLPWGQMSFW >CYTB_bisBis_98A AAW28803 Bison bison NFGSLLGMCLILQILTGLFLAMHYTSDTTTAFSSVAHICRDVNYGWIIRYMHANGASMFFICLYMHAGRGLYYGSYTFLETWNIGVILLLTVMATAFMGYVLPWGQMSFW >CYTB_bisBis_98A_Q322R AAL85955 Bison bison ILTGLFLAMHYTSDTTTAFSSVAHICRDVNYGWIIRYMHANGASMFFICLYMHAGRGLYYGSYTFLETWNIGVILLLTVMATAFMGYVLPWGQMSFWGATVITNLLSAIPYIGTNLVEWIWGGFSVDKATLTRFFAFHFI LPFIIMAIAMVHLLFLHETGSNNPTGISSDMDKIPFHPYYTIKDILGALLLILALMLLVLFTPDLLGDPDNYTPANPLNTPPHIKPEWYFLFAYAILRSIPNKLGGVLALAFSILILALIPLLHTSKQRSMIFRPLSRCLFWTLVADLLTL >CYTB_bosPriW Bos primigenius gi|190360872|gb|ACE76876 MTNFRKSHPLMKIVNNAFIDLPAPSNISSWWNFGSLLGICLILQILTGLFLAMHYTSDTTTAFSSVTHICRDVNYGWIIRYMHANGASMFFICLYMHVGRGLYYGSYTFLETWNIGVILLLTVMATAFMGYVLPWGQMSF WGATVITNLLSAIPYIGTNLVEWIWGGFSVDKATLTRFFAFHFILPFIIMAIAMVHLLFLHETGSNNPTGISSDVDKIPFHPYYTIKDILGALLLILALMLLVLFAPDLLGDPDNYTPANPLNTPPHIKPEWYFLFAYAI LRSIPNKLGGVLALAFSILILALIPLLHTSKQRSMMFRPLSQCLFWALVADLLTLTWIGGQPVEHPYITIGQLASVLYFLLILVLMPTAGTIENKLLKW >CYTB_bosPriM Bos primigenius gi|291463835|gb|ADE05539 alleles F004I A023T I372V MTNIRKSHPLMKIVNNAFIDLPTPSNISSWWNFGSLLGICLILQILTGLFLAMHYTSDTTTAFSSVTHICRDVNYGWIIRYMHANGASMFFICLYMHVGRGLYYGSYTFLETWNIGVILLLTVMATAFMGYVLPWGQMSF WGATVITNLLSAIPYIGTNLVEWIWGGFSVDKATLTRFFAFHFILPFIIMAIAMVHLLFLHETGSNNPTGISSDVDKIPFHPYYTIKDILGALLLILALMLLVLFAPDLLGDPDNYTPANPLNTPPHIKPEWYFLFAYAI LRSIPNKLGGVLALAFSILILALIPLLHTSKQRSMMFRPLSQCLFWALVADLLTLTWIGGQPVEHPYITIGQLASVLYFLLILVLMPTAGTVENKLLKW >CYTB_bosSau Bos sauveli AAV51239 MTNIRKSHPLMKIVNNAFIDLPAPPNISSWWNFGSLLGVCLILQILTGLFLAMHYTSDTTTAFSSVTHICRDVNYGWIIRYMHANGASMFFICLYMHVGRGLYYGSYTFLETWNIGVILLITVMATAFMGYVLPWGQMSF WGATVITNLLSAIPYIGTNLVEWIWGGFSVDKATLTRFFAFHFILPFIIAAIAMVHLLFLHETGSNNPTGVSSDVDKIPFHPYYTIKDTLGALLLILALMLLVLFAPDLLGDPDNYTPANPLNTPPHIKPEWYFLFAYAI LRSIPNKLGGVLALAFSILILILIPLLHTSKQRSMMFRPLSQCLFWTLVADLLTLTWIGGQPVEHPYTTIGQLASIMYFLLILVLMPTAGTVENKLLKW >CYTB_bosfroI Bos frontalis ABO07421 (maternal Bos indicus) MTNIRKSHPLMKIVNNAFIDLPAPSNISSWWNFGSLLGICLILQILTGLFLAMHYTSDTTTAFSSVTHICRDVNYGWIIRYMHANGASMFFICLYMHVGRGLYYGSYTFLETWNIGVILLLTVMATAFMGYVLPWGQMSF WGATVITNLLSAIPYIGTNLVEWIWGGFSVDKATLTRFFAFHFILPFIIMAIAMVHLLFLHETGSNNPTGISSDVDKIPFHPYYTIKDILGALLLILALMLLVLFAPDLLGDPDNYTPANPLNTPPHIKPEWYFLFAYAI LRSIPNKLGGVLALAFSILILALIPLLHTSKQRSMMFRPLSQCLFWALVADLLTLTWIGGQPVEHPYITIGQLASILYFLLILVLMPTAGTVENKLLKW >CYTB_bosFroW Bos frontalis ABO07423 I39V V215A A232T A302I A327T L357M non-hybrid MTNIRKSHPLMKIVNNAFIDLPAPSNISSWWNFGSLLGVCLILQILTGLFLAMHYTSDTTTAFSSVTHICRDVNYGWIIRYMHANGASMFFICLYMHVGRGLYYGSYTFLETWNIGVILLLTVMATAFMGYVLPWGQMSF WGATVITNLLSAIPYIGTNLVEWIWGGFSVDKATLTRFFAFHFILPFIITAIAMVHLLFLHETGSNNPTGISSDADKIPFHPYYTIKDILGTLLLILALMLLVLFAPDLLGDPDNYTPANPLNTPPHIKPEWYFLFAYAI LRSIPNKLGGVLALAFSILILILIPLLHTSKQRSMMFRPLSQCLFWTLVADLLTLTWIGGQPVEHPYITIGQLASIMYFLLILVLMPTAGTVENKLLKW >CYTB_bosGau1 Bos gaurus ADB80894 V39I A62V Y95H T108P L105P T190M N206I ADB80893 ADB80892 EU878387 MTNIRKSHPLMKIVNNAFIDLPAPSNISSWWNFGSLLGVCLILQILTGLFLAMHYTSDTTTAFSSVTHICRDVNYGWIIRYMHANGASMFFICLYMHVGRGLYYGSYTFLETWNIGVILLLTVMATAFMGYVLPWGQMSF WGATVITNLLSAIPYIGTNLVEWIWGGFSVDKATLTRFFAFHFILPFIITAIAMVHLLFLHETGSNNPTGISSDADKIPFHPYYTIKDILGTLLLILALMLLVLFAPDLLGDPDNYTPANPLNTPPHIKPEWYFLFAYAI LRSIPNKLGGVLALAFSILILILIPLLHTSKQRSMMFRPLSQCLFWTLVADLLTLTWIGGQPVEHPYITIGQLASIMYFLLILVLMPTAGTVENKLLKW >CYTB_bosJav Bos javanicus ABS18295 S29A R80W E110K I121F K375N MTNIRKSHPLMKIVNNAFIDLPAPPNISSWWNFGSLLGVCLILQILTGLFLAMHYTSDTTTAFSSVTHICRDVNYGWIIRYMHANGASMFFICLYMHVGRGLYYGSYTFLETWNIGVILLITVMATAFMGYVLPWGQMSF WGATVITNLLSAIPYIGTNLVEWIWGGFSVDKATLTRFFAFHFILPFIITAIAMVHLLFLHETGSNNPTGVSSDADKIPFHPYYTIKDILGALLLILALMLLVLFAPDLLGDPDNYTPANPLNTPPHIKPEWYFLFAYAI LRSIPNKLGGVLALAFSILILILIPLLHTSKQRSMMFRPLSQCLFWTLVADLLTLTWIGGQPVEHPYITIGQLASIMYFLLILVLMPTAGTVENKLLKW >CYTB_bosJav Bos javanicus ABW82495 MTNIRKSHPLMKIVNNAFIDLPAPPNISSWWNFGSLLGVCLILQILTGLFLAMHYTPDTTTAFSSVTHICRDVNYGWIIRYMHANGASMFFICLYMHVGRGLYYGSYTFLETWNIGVILLLTVMATAFMGYVLPWGQMSF WGATVITNLLSAIPYIGTNLVEWIWGGFSVDKATLTRFFAFHFILPFIIMAIAMVHLLFLHETGSNNPTGISSDVDKIPFHPYYTIKDILGALLLILALMLLVLFAPDLLGDPDNYTPANPLNTPPHIKPEWYFLFAYAI LRSIPNKLGGVLALAFSILILALIPLLHTSKQRSMMFRPLSQCLFWILMADLLTLTWIGGQPVEHPYITIGQLASIMYFLLILVLMPTAGTVENKLLKW >CYTB_bosJav Bos javanicus ABW82494 MTNIRKSHPLMKIVNNTFIDLPAPPNISSWWNFGSLLGVCLILQILTGLFLAMHYTSDTTTAFSSVTHICRDVNYGWIIRYMHANGASMFFICLYMHVGRGLYYGSYTFLETWNIGVILLITVMATAFMGYVLPWGQMSF WGATVITNLLSAIPYIGTNLVEWIWGGFSVDKATLTRFFAFHFILPFIITAIAMVHLLFLHETGSNNPTGVSSDADKIPFHPYYTIKDILGALLLILALMLLVLFAPDLLGDPDNYTPANPLNTPPHIKPEWYFLFAYAI LRSIPNKLGGVLALAFSILILILIPLLHTSKQRSMMFRPLSQCLFWTLVADLLTLTWIGGQPVEHPYITIGQLASITYFLLILVLMPTAGTVENKLLKW >CYTB_bosInd Bos indicus ABO07435 T67I sporadic, differs from Bos taurus at I356V and V372I MTNIRKSHPLMKIVNNAFIDLPAPSNISSWWNFGSLLGICLILQILTGLFLAMHYTSDTTTAFSSVTHICRDVNYGWIIRYMHANGASMFFICLYMHVGRGLYYGSYTFLETWNIGVILLLTVMATAFMGYVLPWGQMSF WGATVITNLLSAIPYIGTNLVEWIWGGFSVDKATLTRFFAFHFILPFIIMAIAMVHLLFLHETGSNNPTGISSDVDKIPFHPYYTIKDILGALLLILALMLLVLFAPDLLGDPDNYTPANPLNTPPHIKPEWYFLFAYAI LRSIPNKLGGVLALAFSILILALIPLLHTSKQRSMMFRPLSQCLFWALVADLLTLTWIGGQPVEHPYITIGQLASILYFLLILVLMPTAGTVENKLLKW >CYTB_bisBon Bison bonasus 295065508 YP_003587278 MTNIRKSHPLMKIVNNAFIDLPAPSNISSWWNFGSLLGVCLILQILTGLFLAMHYTSDTTTAFSSVTHICRDVNYGWIIRYMHANGASMFFICLYMHVGRGLYYGSYTFLETWNIGVILLLTVMATAFMGYVLPWGQMSF WGATVITNLLSAIPYIGTNLVEWIWGGFSVDKATLTRFFAFHFILPFIIMAIAMVHLLFLHETGSNNPTG ISSDTDKIPFHPYYTIKDILGALLLILTLMLLVLFAPDLLGDPDNYTPANPLNTPPHIKPEWYFLFAYAI LRSIPNKLGGVLALAFSILILILIPLLHTSKQRSMMFRPLSQCLFWALVADLLTLTWIGGQPVEHPYITIGQLASIMYFLLILVLMPTAGTIENKLLKW >CYTB_bosTau1 Bos taurus AAM12814 208 instances MTNIRKSHPLMKIVNNAFIDLPAPSNISSWWNFGSLLGICLILQILTGLFLAMHYTSDTTTAFSSVTHICRDVNYGWIIRYMHANGASMFFICL YMHVGRGLYYGSYTFLETWNIGVILLLTVMATAFMGYVLPWGQMSFWGATVITNLLSAIPYIGTNLVEWIWGGFSVDKATLTRFFAFHFILPFI IMAIAMVHLLFLHETGSNNPTGISSDVDKIPFHPYYTIKDILGALLLILALMLLVLFAPDLLGDPDNYTPANPLNTPPHIKPEWYFLFAYAILR SIPNKLGGVLALAFSILILALIPLLHTSKQRSMMFRPLSQCLFWALVADLLTLTWIGGQPVEHPYITIGQLASVLYFLLILVLMPTAGTIENKLLKW >CYTB_bosTau2 Bos taurus AAW78524 72 instances V356I I372V MTNIRKSHPLMKIVNNAFIDLPAPSNISSWWNFGSLLGICLILQILTGLFLAMHYTSDTTTAFSSVTHICRDVNYGWIIRYMHANGASMFFICL YMHVGRGLYYGSYTFLETWNIGVILLLTVMATAFMGYVLPWGQMSFWGATVITNLLSAIPYIGTNLVEWIWGGFSVDKATLTRFFAFHFILPFI IMAIAMVHLLFLHETGSNNPTGISSDVDKIPFHPYYTIKDILGALLLILALMLLVLFAPDLLGDPDNYTPANPLNTPPHIKPEWYFLFAYAILR SIPNKLGGVLALAFSILILALIPLLHTSKQRSMMFRPLSQCLFWALVADLLTLTWIGGQPVEHPYITIGQLASILYFLLILVLMPTAGTVENKLLKW >CYTB_synCafW Syncerus caffer 5777912 AAD51426 AF036275 MTHIRKSHPLMKILNNAFIDLPAPSNISSWWNFGSLLGICLILQILTGLFLAMHYTSDTTTAFSSVAHICrDVNYGWIIRYMHANGASMFFICLYMHVGRGLYYGSYTFLETWNIGVILLFTVMATAFMGYVLPWGQMSF WGATVITNLLSAIPYIGTNLVEWIWGGFSVDKATLTRFFAFHFILPFIIAALAMIHLLFLHETGSNNPTGISSDTDKIPFHPYYTIKDILGALLLILALMLLVLFSPDLLGDPDNYTPANPLNTPPHIKPEWYFLFAYAI LRSIPNKLGGVLALILSILILIIMPLLHTSKQRSMMFRPLSQCLFWILVADLLTLTWIGGQPVEHPYIIIGQLASIMYFLLILVLMPTASTIENNLLKW >CYTB_synCafP Syncerus caffer 1813355 BAA11624 H3N T56S I295V MTNIRKSHPLMKILNNAFIDLPAPSNISSWWNFGSLLGICLILQILTGLFLAMHYSSDTTTAFSSVAHICRDVNYGWIIRYMHANGASMFFICLYMHVGRGLYYGSYTFLETWNIGVILLFTVMATAFMGYVLPWGQMSF WGATVITNLLSAIPYIGTNLVEWIWGGFSVDKATLTRFFAFHFILPFIIAALAMIHLLFLHETGSNNPTGISSDTDKIPFHPYYTIKDILGALLLILALMLLVLFSPDLLGDPDNYTPANPLNTPPHIKPEWYFLFAYAI LRSIPNKLGGVLALVLSILILIIMPLLHTSKQRSMMFRPLSQCLFWILVADLLTLTWIGGQPVEHPYIIIGQLASIMYFLLILVLMPTASTIENNLLKW >CYTB_bubBubW Bubalus bubalis ACF17726 MTNIRKSHPLMKILNNAFIDLPAPSNISSWWNFGSLLGICLILQILTGLFLAMHYTSDTTTAFSSVAHICRDVNYGWIIRYMHANGASMFFICLYMHVGRGMYYGSYTFLETWNIGVILLFAVMATAFMGYVLPWGQMSF WGATVITNLLSAIPYIGTSLVEWIWGGFSVDKATLTRFFAFHFILPFIIAALAMVHLLFLHETGSNNPTGISSDTDKIPFHPYYTIKDILGALLLILALMLLVLFTPDLLGDPDNYTPANPLNTPPHIKPEWYFLFAYAI LRSIPNKLGGVLALVLSILILILMPLLHTSKQRSMMFRPFSQCLFWILVANLLTLTWIGGQPVEHPYIIIGQLASITYFLLILVLMPTASMIENNLLKW >CYTB_bubBubP Bubalus bubalis ABR08397 MTNIRKSHPLMKILNNAFIDLPAPSNISSWWNFGSLLGICLILQILTGLFLAMHYTSDTTTAFSSVAHICRDVNYGWIIRYMHANGASMFFICLYMHVGRGMYYGSYTFLETWNIGVILLFAVMATAFMGYVLPWGQMSF WGATVITNLLSAIPYIGTSLVEWIWGGFSVDKATLTRFFAFHFILPFIIAALAMVHLLFLHETGSNNPTGISSDTDKIPFHPYYTIKDILGALLLILALMLLVLFAPDLLGDPDNYTPANPLNTPPHIKPEWYFLFAYAI LRSIPNKLGGVLALVLSILILILMPLLHTSKQRSMMFRPFSQCLFWILVANLLTLTWIGGQPVEHPYIIIGQLASITYFLLILVLMPTASMVENNLLKW >CYTB_traScr1 Tragelaphus scriptus AF036277 MTNIRKSHPLMKIVNNAFIDLPAPSNISSWWNFGSLLGICLILQILTGLFLAMHYTSDTMTAFSSVTHICRDVNHGWIIRYMHANGASMFFICLYMHVGRGMYYGSYTFLETWNIGVILLFTVMATAFMGYVLPWGQMSF WGATVITNLLSAIPYIGTSLVEWIWGGFSVDKATLTRFFAFHFILPFIIAALAMVHLLFLHETGSNNPTGIPSDMDKIPFHPYYTIKDILGALLLILILMLLVLFAPDLLGDPDNYTPANPLNTPPHIKPEWYFLFAYAI LRSIPNKLGGVLALVFSILILILMPLLHTSKQRSMMFRPLSQCLFWILAADLLTLTWIGGQPVEHPYIIIGQLASIMYFLIILVLMPATSMIENSFLKW >CYTB_traScr2 Tragelaphus scriptus AAD13501 MTNIRKSHPLMKIVNNAFIDLPAPSNISSWWNFGSLLGICLILQILTGLFLAMHYTWDTMTAFSSVTHICRDVNYGWIIRYMHANGASMFFICLYMHVGRGMYYGSYTFLETWNIGVILLFTVMATAFMGYVLPWGQMSF WGATVITNLLSAIPYIGTSLVEWIWGGFSVDKATLTRFFAFHFILPFIIAALAMVHLLFLHETGSNNPTGIPSDMDKIPFHPYYTIKDILGALLLILILMLLVLFAPDLLGDPDNYAPANPLNTPPHIKPEWYFLFAYAI LRSIPNKLGGVLALVLSILILILMPLLHTSKQRSMMFRPLSQCLFWILAADLLTLTWIGGQPVEHPYIIIGQLASIMYFLIILVLMPAVSMIENNLLKW >CYTB_traScr3 Tragelaphus scriptus non-sporadic alleles S159N A190T M205T A232V L234M I238T V243T F296L I302V lower case MTNIRKSHPLMKIVNNAFIDLPAPSNISSWWNFGSLLGICLILQILTGLFLAMHYTSDTMTAFSSVTHICRDVNHGWIIRYMHANGASMFFICLYMHVGRGMYYGSYTFLETWNIGVILLFTVMATAFMGYVLPWGQMSF WGATVITNLLSAIPYIGTnLVEWIWGGFSVDKATLTRFFAFHFILPFIItALAMVHLLFLHETGSNNPTGIPSDtDKIPFHPYYTIKDILGvLLLILtLMLLtLFAPDLLGDPDNYTPANPLNTPPHIKPEWYFLFAYAI LRSIPNKLGGVLALVlSILILvLMPLLHTSKQRSMMFRPLSQCLFWILAADLLTLTWIGGQPVEHPYIIIGQLASIMYFLIILVLMPATSMIENSFLKW >CYTB_traEur Tragelaphus eurycerus (bongo) AAD51427 MINIRKSHPLMKIVNNAFIDLPAPSNISSWWNFGSLLGICLILQILTGLFLAMHYTSDTTTAFSSVTHICRDVNYGWIIRYMHANGASMFFICLYMHVGRGMYYGSYTFLETWNIGVILLFTVMATAFTGYVLPWGQMSF WGATVITNLLSAIPYIGTSLVEWIWGGFSVDKATLTRFFAFHFILPFIITALAMVHLLFLHETGSNNPTGISSNMDKIPFHPYYTIKDILGALLLILTLMLLVLFAPDLLGDPDNYTPANPLNTPPHIKPEWYFLFAYAI LRSIPNKLGGVLALVLSILILILMPLLHMSKQRSMMFRPLSQCLFWILAADLLTLTWIGGQPVEHPYIIIGQLASIMYFLIILVLMPVTSMIENNFLKW >CYTB_traStr Tragelaphus strepsiceros (greater kudu) AAD51431 MTNIRKSHPLMKIVNNAFIDLPAPSNISSWWNFGSLLGICLILQILTGLFLAMHYTSDTTTAFSSVTHICRDVNYGWIIRYMHANGASMFFICLYVHVGRGMYYGSYTFLETWNIGVILLFTVMATAFMGYVLPWGQMSF WGATVITNLLSAIPYIGTNLVEWIWGGFSVDKATLTRFFAFHFILPFIIAALAMVHLLFLHETGSNNPTGISSDMDKIPFHPYYTIKDILGALLLVLALMLLVLFTPDLLGDPDNYTPANPLNTPPHIKPEWYFLFAYAI LRSIPNKLGGVLALVLSILILIFLPLLHTSKQRSMMFRPLSQCLFWILVADLLTLTWIGGQPVEHPYMIIGQLASIMYfLLILVLMPVTSMIENNFLKW >CYTB_traImb Tragelaphus imberbis (lesser kudu) AAD13498 MINIRKSHPLMKIVNNAFIDLPTPPNISSWWNFGSLLGICLVLQILTGLFLAMHYTSDTMTAFSSVTHICRDVNYGWIIRYMHANGASMFFICLYMHVGRGLYYGSYTFLETWNIGVILLFTVMATAFMGYVLPWGQMSF WGATVITNLLSAIPYIGTNLVEWIWGGFSVDKATLTRFFAFHFILPFIIAALALVHLLFLHETGSNNPTGISSDTDKIPFHPYYTIKDILGALLLILALMLLVLFAPDLLGDPDNYTPANPLNTPPHIKPEWYFLFAYAI LRSIPNKLGGVLALILTILMPILMPLLHASKQRSMMFRPLSQCLFWILVADLLTLTWIGGQPVEHPYIIIGQLASIMYFLLILVLMPMAGSIENNLLKW >CYTB_traOry Tragelaphus oryx (eland) AAD13491 MTNIRKSHPLMKIVNNAFIDLPTPSNISSWWNFGSLLGICLTLQILTGLFLAMHYTSDTTTAFSSVTDICRDVNYGWIIRYMHANGASMFFICLYMHVGRGMYYGSYTFLETWNIGVILLFTVMATAFMGYVLPWGQMSF WGATVITNLLSAIPYIGTSLVEWIWGGFSVDKATLTRFFAFHFILPFIIAALAMVHLLFLHETGSNNPTGISSDTDKIPFHPYHTIKDILGALLLILTLMLLVLFAPDLLGDPDNYTPANPLNTPPHIKPEWYFLFAYAI LRSIPNKLGGVLALVLSILILILMPLLHTSKQRSMMFRPLSQCLFWVLAADLLTLTWIGGQPVEHPYIIIGQLASIMYFLLILVLMPVASMIENNFL >CYTB_traAng Tragelaphus angasii (nyala) AAD42706 MTNIRKSHPLMKIVNNAFIDLPAPSNISSWWNFGSLLGVCLILQILTGLFLAMHYTSDTMTAFSSVTHICRDVNYGWIIRYMHANGASMFFICLYMHVGRGLYYGSYTFLETWNVGVILLFMVMATAFMGYVLPWGQMSF WGATVITNLLSAIPYIGTNLVEWIWGGFSVDKATLTRFFAFHFILPFIITALVMVHLLFLHETGSNNPTGISSDMDKIPFHPYYTIKDILGALLLILALMVLVLFTPDLLGDPDNYTPANPLNTPPHIKPEWYFLFAYAI LRSIPNKLGGVLALVLSILILILMPLLHMSKQRSMMFRPLSQCLFWLLVADLLTLTWIGGQPVEHPYIIIGQLASIIYFLLILVLMPVISTIENNLLKW >CYTB_traSpi Tragelaphus spekii (sitatunga) CAA10935 MTNIRKSHPLMKIVNNAFIDLPAPSNISSWWNFGSLLGICLILQILTGLFLAMHYTSDTTTAFSSVTHICRDVNYGWIIRYMHANGASMFFICLYMHVGRGMYYGSYTFLETWNIGVILLFTVMATAFMGYVLPWGQMSF WGATVITNLLSAIPYIGTSLVEWIWGGFSVDKATLTRFFAFHFIFPFIIAALAMVHLLFLHETGSNNPTGISSDMDKIPFHPYYTIKDILGVLLLILTLMLLVLFAPDLLGDPDNYTPANPLITPPHIKPEWYFLFAYAI LRSIPNKLGGVLALVLSILILILMPLLHVSKQRSMMFRPLSQCLFWILAADLLTLTWIGGQPVEHPYIIIGQLASIMYFLIILVLMPATSMIENNFLKW >CYTB_traDer Taurotragus derbianus (giant eland) AAD13496 MTNIRKSHPLMKIVNNAFIDLPAPSNISSWWNFGSLLGICLILQILTGLFLAMHYTSDTTTAFSSVTHICRDVNYGWIIRYMHANGASMFFICLYMHVGRGMYYGSYTFLETWNIGVILLFTVMATAFMGYVLPWGQMSF WGATVITNLLSAIPYIGTSLVEWIWGGFSVDKATLTRFFAFHFILPFIIAALAIVHLLFLHETGSNNPTGISSDMDKIPFHPYYTIKDILGALLLILALMLLVLFAPDLLGDPDNYTPANPLSTPPHIKPEWYFLFAYAI LRLIPNKLGGVLALVLSILVLMLMPLLHTSKQRSMMFRPLSQCFFWILAADLLTLTWIGGQLVEHPYIIIGQLASIMYFLLILVLMPVASMIENNLLKW >CYTB_bseTra Boselaphus tragocamelus CAA10934 MTNIRKSHPLMKIVNNAFIDLPAPSNISSWWNFGSLLGICLILQILTGLFLAMHYTSDTMTAFASVTHICRDVNYGWIIRYMHANGASMFFICLYMHVGRGLYYGSYTFLETWNIGVILLFTVMATAFMGYVLPWGQMSF WGATVITNLLSAIPYIGTNLVEWIWGGFSVDKATLTRFFAFHFILPFIIAALAMIHLLFLHETGSNNPTGISSDADKIPFHPYYTIKDILGALLLILALMMLVLFAPDLLGDPDNYTPANPLSTPPHIKPEWYFLFAYAI LRSIPNKLGGVMALVLSILILILMPLLHTSKQRSMMFRPLSQCMFWILVANLLTLTWIGGQPVEHPYIIIGQLASIMYFLLILVLMPTASMIENNLLKW
Interpreting yak CYTB variation
Yaks are the closest living sister species to bison. Although 15,000 wild yaks still persist, they have been subject to very similar pressures to those experienced by bison: bottlenecks, population fragmentation, introgression from long domesticated yaks and hybridization with cattle. Adaptations specific to mitochondria may exist as yak live at altitudes exceeding 4000 meters with average annual temperatures in rearing areas –8°C, with animals surviving winter temperatures of –40°C.
Because yaks provide the immediate outgroup for bison genetics (and vice versa), their parallel mitochondrial proteomics are investigated in depth here. This further enables reconstruction of mitochondrial proteins of their last common ancestor (after consideration of lineage sorting) and correct placement of Pleistocene genomic sequences.
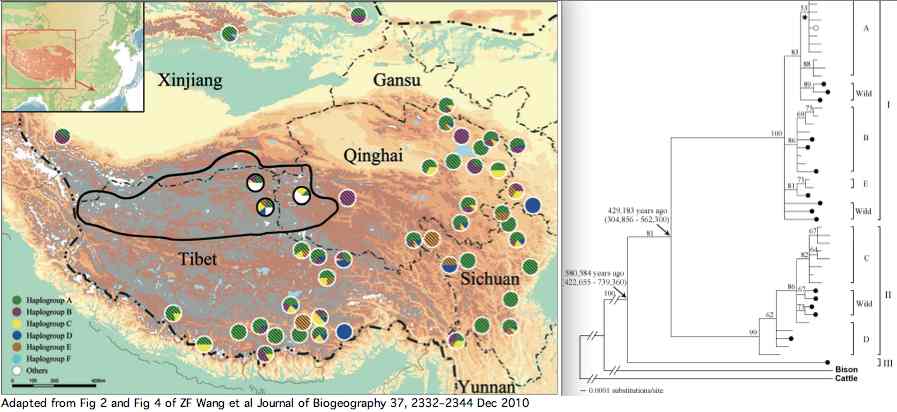
Data availability for yaks was greatly improved by a Dec 2010 paper by Zhaofeng Wang et al. that investigated yak phylogeographical structure and demographic history on the Qinghai-Tibetan Plateau. Complete mitochondrial genomes were determined for 48 domesticated and 21 wild yaks. The three lineages shown in article supplemental established diverged at 420 kyr and 580 kyr in accordance with extended but temporary allopatric migration barriers created by two large plateau glaciations.
The wild yaks are found in all three branches of the tree (solid circles in figure). Their entries at GenBank are distinguished by a W (for wild) prefix, eg isolate W77 GQ464266. There is potential for confusion here because NCBI taxonomy uses Bos grunniens mutus for wild yak, yet the subspecies concept is contradicted by the mixed distribution of wild and domestic yaks in the mitochondrial tree. Related taxa such as Bos mutus (Przewalski, 1883), Bos mutus grunniens, and Poephagus mutus also conflict with the facts. Yak and bison -- diverging at 2.5 million years -- need to reside in the same genus.
The primary focus here are protein polymorphisms in wild yak because domesticated animals may exhibit inbreeding issues and other evolutionary artifacts due to their estrangement from darwinian selection. Consequently it is important to track which GenBank entries reference wild yaks.
Bos grunniens mutus has three GenBank entries relevant to cytochrome b: proteins AAX53006 and AY955226 both containing unique V195A, I348F mutations in an otherwise wildtype background and CAA76015, an older fragmentary wildtype sequence not considered further here. The first two animals add samples to the large, remote Xinjiang province but remain unpublished (Liu,Q Wu,M Li,Y) despite the 27 Mar 2005 submission date at GenBank. (A number of D-loop sequences submitted for this taxon on 19 Jan 2009 by 27-MAR-2005 by Ma,ZJ also remain unpublished.)
The Myanmar/Bhutan mithun sequence BAJ05329 attributed to Bos grunniens at GenBank has 12 differences to wild yak but is 100% identical to 94 Bos indicus entries, ie it is a hybrid and its mitochondrial genome is irrelevant here. Such GenBank errors are all but impossible to correct.
The 21 new genome accessions of wild yak are GQ464266, GQ464265, GQ464264, GQ464263, GQ464262, GQ464261, GQ464260, GQ464259, GQ464258, GQ464257, GQ464256, GQ464255, GQ464254, GQ464253, GQ464252, GQ464251, GQ464250, GQ464249, GQ464248, GQ464247, GQ464246. These were not mapped to the published tree.
In terms of protein accessions (which will be shown at NCBI blastp output), these are ACU81659, ACU81646, ACU81633, ACU81620, ACU81607, ACU81594, ACU81581, ACU81568, ACU81555, ACU81542, ACU81529, ACU81516, ACU81503, ACU81490, ACU81477, ACU81464, ACU81451, ACU81438, ACU81425, ACU81412, ACU81399 to which AAX53006 and AY955226 can be added.
Of these, 16 fall in the main reference sequence group (wildtype) but 5 wild plateau yaks exhibit polymorphisms that cannot be attributed to domestication. As noted, two additional wild yaks from extreme NW China have additional double mutations but no associated PubMed publication nor tissue source indicated. As either change alone would inactivate an essential enzyme, these represent either heteroplasmic oddities or sequence error (to be pursued as other proteins are considered). The remaining sequences were derived from muscle and skin dna.
There is no overlap between wild yak polymorphism sites and the five of domestic yak. Alleles occurring in full length sequences are analyzed further below.
The summary table of yak CYTB amino acid polymorphisms below arises from alignment of 5000 full-length mammalian cytochrome b orthologs. Magenta indicates a deleterious change at an invariant position,red a deleterious mutation at a naturally polymorphic site, green a possibly acceptable change but of restricted distribution and fitness, and blue a near-neutral substitution. Gray is reserved for probable sequencing error. It can be seen that the smallish yak population sampled (72 animals) already contains 5 deleterious alleles in CYTB which represents only 10% of the amino acids of the mitochondrial proteome.
In summary, out of 70 individual yaks, 10 are carrying deleterious mutations at five sites. That seems like an extraordinary number for a central enzyme in energy metabolism for which it is difficult to envision compensation by another gene. Restricting to the 21 wild yaks, 3 have deleterious polymorphism and 1 has a marginal change. Overall 1 in 7 animals is affected just in this one gene. However CYTB is but one of 13 encoded by the mitochondrial genome -- what sort of genetic burden are yaks carrying overall?
1 ACU81568 A017T wild yak isolate W50 GQ464259 2 ACU81399 I192T wild yak isolate W02 GQ464246 ACU81633 I192T wild yak isolate W75 GQ464264 3 ACU81555 D214N wild yak isolate W40 GQ464258 4 AAX53006 V195A I348F mutus isolate Xinjiang01 unpublished Liu,Q Wu,M Li,Y AAX53007 V195A I348F mutus isolate Xinjiang02 unpublished Liu,Q Wu,M Li,Y 5 ACU81529 V329M wild yak isolate W1313 GQ464256 6 ABI15999 V039I A067T domestic yak fragment PUBMED:17257194 Poephagus 7 ABI16000 V039I A067T domestic yak fragment PUBMED:17257194 Poephagus ACU82153 A084T domestic yak isolate HY5 8 ACU82101 V098L domestic yak isolate HY1 9 AAU89116 I118T domestic yak =SP:Q5Y4Q0 PUBMED:16942892 ACU81711 I118T domestic yak isolate HZ3 ACU81737 I118T domestic yak isolate MQ1 AAS93096 I118T domestic yak fragment PUBMED:17257194 AAS93099 I118T domestic yak fragment PUBMED:17257194
Although the mitochondria encodes the usual 20 amino acids, only a subset of chemically similar residues ever appear at a given position in a given protein -- its reduced alphabet. This subset describes the evolutionarily acceptable substitutions that do not significantly disrupt protein functionality. Discovery of this reduced alphabet can be achieved with greater precision the higher the number of available species and individual sequences multiplicities. For mitochondrial proteins, that sensitivity is 1 in 10,000 (0.01% occurrence frequency) for a given amino acid, much better than even the much-studied human nuclear genome.
Interpretive certainty is never attained without experimentation (yeast is a surprisingly informative model system) but improves up to a point with more sequence data. Here it is important to check whether less common substitutions have persisted over evolutionary time in a phylogenetically coherent manner (ie a sub-clade) or are novel adaptations perhaps in conjunction with a co-evolving residue at another site (or another protein, perhaps nuclear-encoded). After these considerations, the remaining rare changes are mostly deleterious (or sequencing error) but rarely adaptive. Polymorphism significance can be pursued at the xray structural level for only 3 of the 13 mitochondrial proteins (CYTB, COX2, COX1) and even this is complicated in the case of CYTB by its oligomeric association with 3 nuclear encoded proteins.
Aligning CTYB from the 72 complete yak mitochondrial genomes available on 1 Dec 10 shows variation at just 9 sites along the protein (ie 9 nsSNPs). These are quickly found when the web alignment tool retains input sequence order, displays residues identical to the top sequence as dots, gaps fragmentary data correctly, and allows a wide display permitting effective cross-species comparisons.
Yak and bison -- despite being sister species -- share variation only at one site, position 98. Here yak is exclusively valine with the exception of a single deleterious occurrence (see below) of leucine, whereas bison have a mix of valine and alanine (which otherwise is very rare at this position in mammals), ie the ancestral residue was valine. Thus no lineage sorting occurred at any amino acid position in CYTB at the time these two species diverged at 2.5 myr. Lineage sorting however may be important in the overall evolution of the Bovini: 53 ancient polymorphisms (at the dna level) are said to have persisted since Bos and Bison diverged from Bubalus 5–8 million years ago.
The changes can also be displayed in context by coloring the appropriate residues in a reference sequence relative to a composite sequence consolidating all the polymorphisms from distinct animals (no one animal has more than two of the 9; V195A + I348F occurs in two animals). The composite sequence is quite useful in comparing polymorphism sites across species as explained in the annotation tricks section.
>CYTB_bosGruR Bos grunniens cytochrome b ref seq taken as gi|147744503
MTNIRKSHPLMKIVNNAFIDLPAPSNISSWWNFGSLLGVCLILQILTGLFLAMHYTSDTTTAFSSVAHICRDVNYGWIIRYMHANGASMFFICLYMHVGRGLYYGSYTFLETWNIGVILLLTVMATAFMGYVLPWGQMSF
WGATVITNLLSAIPYIGTNLVEWIWGGFSVDKATLTRFFAFHFILPFIITAIAMVHLLFLHETGSNNPTGISSDADKIPFHPYYTIKDILGALLLILALMLLVLFTPDLLGDPDNYTPANPLNTPPHIKPEWYFLFAYAI
LRSIPNKLGGVLALAFSILILALIPLLHTSKQRSMIFRPLSQCLFWTLVADLLTLTWIGGQPVEHPYIIIGQLASIMYFLLILVLMPTAGTIENKLLKW
>CYTB_bosGruP Bos grunniens composite polymorphisms: A017T A084T V098L I188T I192T V195A D214N V329M I348F
MTNIRKSHPLMKIVNNTFIDLPAPSNISSWWNFGSLLGVCLILQILTGLFLAMHYTSDTTTAFSSVAHICRDVNYGWIIRYMHTNGASMFFICLYMHLGRGLYYGSYTFLETWNIGVTLLLTVMATAFMGYVLPWGQMSF
WGATVITNLLSAIPYIGTNLVEWIWGGFSVDKATLTRFFAFHFILPFIITATAMAHLLFLHETGSNNPTGISSNADKIPFHPYYTIKDILGALLLILALMLLVLFTPDLLGDPDNYTPANPLNTPPHIKPEWYFLFAYAI
LRSIPNKLGGVLALAFSILILALIPLLHTSKQRSMIFRPLSQCLFWTLMADLLTLTWIGGQPVEHPYFIIGQLASIMYFLLILVLMPTAGTIENKLLKW
wild dom dom dom wild dom wild wild dom
A017T A084T V098L I118T I192T V195A D214N V329M I348F
4018S 4994A 4522V 4309I 4353L 4528V 4429D 4610V 4232I
927A* 3T 430I 667S 505M 427I 512N 188T 651V
46T 1P 34M 14I 94I* 25T 43E 133A 63T
3L 1V 11A 1T 31T 4G 8S 44I 45M
3M 1L 3F 4M 2Y 22M 4N
1F 1N 2V 1A 1H 2G 2F
1P 1A 1E 1A
1S
A017T: At position 98, the mammalian reduced alphabet consists primarily of serine but with yak alanine also well represented at 18%. Threonine occurs in 46 sequences so cannot be sequence error or serious mutation. Bulk seems to be the main criterion at this site rather than polarity -- threonine though polar is bulkier residue than serine or alanine. To determine whether it has arisen multiple times or just in one clade, the phylogenetic distribution of the 46 occurrences needs consideration.
It can be seen from the graphic at left that A017T has arisen multiple times with no common denominator (such as high elevation lifestyle) but -- with the exception of monotremes -- never in a deep stem ancestor. That is, A017T occurs here and there but only in recently speciated clades. This suggests that while not lethal, over time it gets replaced by more adaptive serine or alanine.
A017T
4018 S
927 A yak do not have the most common amino acid at position 17
46 T
3 L
3 M
1 F
1 P
A084T: At position 84, alanine is strictly invariant. Thus threonine is an unmistakable deleterious mutation in domestic yak.
A084T
4994 A
3 T
1 P
1 V
V098L: At position 98, the reduced alphabet consists of valine 90% of the time regardless of mammalian clade with the similar (branched chain aliphatic) isoleucine having substantial dispersed representation at nearly 9%. The 430 species in which it occurs are scattered incoherently within mammal clades, meaning that it has arisen independently many times. V098I may be slightly suboptimal as there is an evident bias (at some level) against equal occurrence. It likely co-exists with valine in most non-bottlenecked populations of mammals, observed if enough individuals of a given species are sequenced.
However leucine, the seemingly similar third aliphatic residue, occurs one once despite being but a single base change transition away from the dominant residue. Were leucine a near-neutral substitution, its incidence would be vastly higher. Thus the change V098L reported for yak represents either a deleterious mutation or an unprecedented adaptation (eg to high altitude) or sequencing error in GenBank entry ACU82101. The same can be said for the more overtly radical change V098N in lemur AAS00156. The 34 methionines occur sporadically in the phylogenetic tree suggesting they are sub-adaptive and blink out over time. Indeed, canine spongiform leukoencephalomyelopathy is attributed to V98M. Dog CYTB is 89% identical to that of yak and numbering corresponds.
V098L 4522 V 430 I 34 M 11 A bison 1 L yak 1 N lemur
I118T: At position 118, the reduced alphabet consists predominantly of ILV with some A and M, a very common occurrence proteomewide. TSF are all deleterious mutations in domestic yak.
I118T
2597 I
1843 L
404 V
87 A
61 M
6 T (all yak)
1 S
1 F
I192T: At position 192 of wild yak, the dominant residue is leucine instead of the yak ancestral value isoleucine, which is disfavored relative to methionine, ie isoleucine is a mild polymorphism in its own right but the associated taxonomy shows it narrowly restricted to 83 sequences in Bos, Bison, and separately in 5 Kobus (waterbucks), too persistent to be dysfunctional and indeed a candidate for adaptive. However change to polar threonine is seen in 31 nominal species but after removal of redundancy, only in two species of pocket mice. Thus the yak change is deleterious.
I192T
4353 L
505 M
94 I
31 T
3 F
2 V
1 A
1 S
V195A: This allele occurs together with I348F in two wild yaks from a remote region in NW China. Despite sequence submission, no article has appeared in the three subsequent years. It can be seen from the reduced alphabet frequencies that this is a severe mutation (as is I348F) so taken together likely sequence just error. No further analysis will be done here until such time as the polymorphisms are confirmed.
V195A
4528 V
427 I
25 T
4 G
4 M
1 A
D214N: This polymorphism of wild yak is seen quite widely, in some 10% of mammals. The 223 taxa with D214N are mostly confined to laurasiatheres and glires but are not a hallmark of these clades. Nor do the species with asparagine have any common lifestyle denominator. Asparagine is an acceptable variation for aspartate at this site if perhaps not optimal.
D214N
4429 D
512 N
43 E
8 S
4 X
2 Y
1 H
V329M: This allele occurs in wild yak. Methionine is not a radical substitution in terms of physical/chemical properties and similar additional amino acids appear at low levels, even though valine occurs in a huge majority of species. Methionine occurs in 17 other species phylogenetically scattered species include Bos javanicus, Ovis, Budorcas, Naemorhedus, Mus, Rattus, bats and sloth. Thus it is likely suboptimal but not significantly deleterious.
V329M
4610 V
188 T
133 A
44 I
22 M
2 G
1 E
I348F: This allele occurs together with V195A in two wild yaks from a remote region in NW China. Despite sequence submission, no article has appeared in the three subsequent years. In can be seen from the reduced alphabet frequencies that this is a severe mutation but more likely sequence error, as is V195A.
I348F
4232 I348F
651 V
63 T
45 M
4 N
2 I348F
1 A
Human CYTB polymorphism and disease
Polymorphisms and pathogenic mutations disease for human CYTB have been very helpfully compiled by mtDB and MitoMap, with other mammals at OMIA. Fortunately, numbering systems carry over without change to bison and yak since no indels occur in this gene within mammals.
A poor tradition in mitochondrial research allows amino acid changes to be described just by a single nucleotide coordinate relative to the Cambridge Reference Sequence, NC_012920. That requires the user to have a numbered translation via the mitochondrial genetic code showing in-frame amino acids; however the change from the protein perspective (eg V98T) is often conveniently displayed at Uniprot. Coordinates for all mitochondrial features are tabulated here; CYTB extends from position 14747-15887.
Given over 7000 complete human mitochondrial genomes and a high mutation rate, some human polymorphic sites will inevitably overlap with yak and bison alleles. Thus any information about associated human disease at the 16 known disease sites might be transferable. However many rare and obviously dysfunctional alleles were collected for population haplotype mapping and no disease information was collected.
Annotation transfer is vastly complicated by heteroplasmy, experimentalist inability to establish the heritability of the allele, and differences in tissues used to obtain dna for sequencing and so neglects the possibly compensatory effect of changes elsewhere in this gene (or nuclear genes that interact with it), a substantial issue in a protein like cytochrome b where 10% of the residues between bovids and human are non-identical. At such sites (eg H214Y human, D214N yak), transfer of phenotypic information is dubious.
brown in the human allele table below indicates human polymorphisms corresponding to an allele of concern in yak or bison. In two significant cases -- both in domestic yak -- the initial and final residue of human are identical to that of yak, namely A084T* and I118T*. Both are predicted to be deleterious in both human and yak. Unfortunately no clinical information was collected on the human side and the health status of the yaks is unknown (eg level of exercise intolerance).
However even A084T (a strongly invariant site in all mammals) was evidently not early-lethal for its adult human carrier (dna samples are collected from adult volunteers whose health status is not recorded). Here the vast and still unsettled complexities of mitochondrial genomics may come into play:
- a single mitochondrion may up to 10 replicated copies of its genome which need not be identical
- cells can carry thousands of mitochondria inherited erratically during embryogenesis and later stem cells
- dna samples, not being collected from germline cells, may represent non-heritable somatic mutations in restricted descendent cells of the tissue sampled
- disease onset is often in late adulthood due to the nature of mitochondrial replication and dispersal to daughter cells and so may not be applicable to shorter-lived species
Symptoms of severe heteroplasmic mitochondrial disorders frequently do not appear until adulthood because many cell divisions and much time is required for a cell to receive enough mitochondria containing the mutant alleles to cause symptoms. An example of this phenomenon is Leber optic atrophy (LHON). Affected individuals may not experience vision difficulties until they have reached adulthood. Another example is MERRF syndrome (Myoclonic Epilepsy with Ragged Red Fibers). Heteroplasmy here explains the variation in severity of the disease among siblings. The incidence of heteroplasmy in human mtDNA is unknown, as the number of individuals who have been subjected to mtDNA testing for reasons other than the diagnosis of mitochondrial disorders is small."
The oft-observed disease Leber Hereditary Optic Neuropathy (LHON) is genetically heterogeneous, arising from mutations in other mitochondrial genes (R340H in ND4, A52T in ND1 and M64V in ND6, subunits of complex I of the oxidative phosphorylation chain in mitochondria) as well as from CYTB variants A29T and secondarily D171N and V356M.
tRNA disruptions in bison were analyzed by Douglas et al. Here it is known the human disease MERRF disrupts mitochondrial tRNA-Lys in 80% of cases and so biosynthesis of mitochondrial proteins essential for oxidative phosphorylation. It too is genetically heterogeneous as tRNAs for leucine, histidine, serine and phenylalanine can be affected in other individuals.
Mitochondrial diseases arise frequently: 1 in 4000 individuals is at risk of developing a mitochondrial disease sometime in their lifetime. Half of those affected are children who show symptoms before age five, and approximately 80% of them will die before age 20. The mortality rate is roughly that of cancer... The mutation rate of the mitochondrial genome is 10–20 times greater than of nuclear DNA, and mtDNA is more prone to oxidative damage than is nuclear DNA. Mutations in human mtDNA cause premature aging, severe neuromuscular pathologies and maternally inherited metabolic diseases, and influence apoptosis.
human yak A084T A084T* seen twice in Japanese population I098V V098L I118V I118T I118T I118T* seen once in Japan and once in India H214Y D214N A329T V329M T2A S56A I117V D171N I211T G251S M316T A354T T2I S56L I118V D171G T212A E251D Y325H V356M M4V T61A I118T S172N T212I Y256H A329T V356A M4T T70A L121F P173S H214Y T257I A330T T360A R5G Y75C A122T T174A T219A L258P A330V T360M I7T I78V T123A F181L T219I A259T I334V T368A N8S I78T A125T I184V I226V N260D T336A T368I N15S L82F E136D L185S A229T V284I I338V I369V H16R A84T F140L I189V L230F V291A P342S I369T F18L G86S L149M A190T L233V S297P V343M I372V I19M C93Y I153T A190V F235L I300T V343A M376V A29T I98V Y155H A191T L236I I304T S344G A380T A39T G101S I156V A191D S238P I306V S344N A380V A39V Y109H I156T A193T S238F I306T Y345F ---- I42V E111K T158A T194A T241A M309V T348I ---- I42T T112A D159N T194V T241M M309T I349V ---- F50L W113R I164V F199L T243A S310P I349T ---- F50L I115T G167S I211V F245L M316V V353M ---- Of known disease mutations, only V98M corresponds to a bison allele: A29T LHON Leber hereditary optic neuropathy G34S mitochondrial myopathy; sporadic S35P exercice intolerance V98M* dog leukoencephalomyelopathy S151P exercise intolerance G166E hyperthrophic cardiomyopathy D171N secondary LHON G231D 16026996 mouse G251D CMIH G251S obesity N255H cardiomyopathy Y278C multisystem disorder G290D exercise intolerance S297P neonatal polyvisceral failure G339E mitochondrial myopathy V356M secondary LHON
- See abstracts for all 16 disease sites
- Adaptive rates of evolution in all 13 genes from an alignment of 214 mammalian mitochondrial genomes
- Adaptive evolution of the mammalian mitochondrial genome
- A series of 12 papers on mitochondrial dna inheritance issues
Cytochrome b mutations in Leber hereditary optic neuropathy CYTB:D171N CYTB:V356M ND5:A458T New mutations were discovered in the apocytochrome b gene in Leber hereditary optic neuropathy probands who did not harbor either of the two known Complex I mutations (positions 3,460 and 11,778). A mutation at position 15,257 was found in eight independent probands which changed a highly conserved D to N, was not found in controls, and appears to be pathogenetically significant. The 15,257 mutation occurred in association with a known synergistic mutation at position 13,708 in 7/8 probands (ie ND5 A458T) and in association with a new apocytochrome b mutation at position 15,812 (ie V356M) in 4/8 probands. Mutations in Complex III genes may be involved in Leber hereditary optic neuropathy and multiple, simultaneous mutations occur frequently.
Mazunin IO (2010) Mitochondrial genome and human mitochondrial diseases. Molecular Biology 44(5) Today there are described more than 400 point mutations and more than hundred of structural rearrangements of mitochondrial DNA associated with characteristic neuromuscular and other mitochondrial syndromes, from lethal in the neonatal period of life to the disease with late onset. The defects of oxidative phosphorylation are the main reasons of mitochondrial disease development. Phenotypic diversity and phenomenon of heteroplasmy are the hallmark of mitochondrial human diseases. It is necessary to assess the amount of mutant mtDNA accurately, since the level of heteroplasmy largely determines the phenotypic manifestation. In spite of tremendous progress in mitochondrial biology since the cause-and-effect relations between mtDNA mutation and the human diseases was established over 20 years ago, there is still no cure for mitochondrial diseases.
Pathogenic mitochondrial DNA mutations in protein-coding genes
Lee-Jun C. Wong PhD Muscle Nerve, 2007
More than 200 disease-related mitochondrial DNA (mtDNA) point mutations have been reported in the Mitomap (http://www.mitomap.org) database. These mutations can be divided into two groups: mutations affecting mitochondrial protein synthesis, including mutations in tRNA and rRNA genes; and mutations in protein-encoding genes (mRNAs). This review focuses on mutations in mitochondrial genes that encode proteins. These mutations are involved in a broad spectrum of human diseases, including a variety of multisystem disorders as well as more tissue-specific diseases such as isolated myopathy and Leber hereditary optic neuropathy (LHON). Because the mitochondrial genome contains a large number of apparently neutral polymorphisms that have little pathogenic significance, along with secondary homoplasmic mutations that do not have primary disease-causing effect, the pathogenic role of all newly discovered mutations must be rigorously established. A scoring system has been applied to evaluate the pathogenicity of the mutations in mtDNA protein-encoding genes and to review the predominant clinical features and the molecular characteristics of mutations in each mtDNA-encoded respiratory chain complex.
S297P homoplasmic in all tissues tested, undetectable in mother PMID: 19563916
Eur J Hum Genet. 2004 Mar;12(3):220-4.
The deleterious G15498A mutation in mitochondrial DNA-encoded cytochrome b may remain clinically silent in homoplasmic carriers.
We report on a patient with severe growth retardation and IgF1 deficiency, in which a mitochondrial abnormality was suspected. An isolated mitochondrial respiratory chain complex III deficiency was found in blood lymphocytes and skin fibroblasts. Sequence analysis of the cytochrome b, which is the only mitochondrial DNA-encoded subunit of complex III, revealed a homoplasmic G15498A mutation, resulting in the substitution of a highly conserved amino acid (glycine 251 into an aspartic acid). The mutation was found to be homoplasmic in all tissues examined from the mother and her brother (lymphocytes, fibroblasts, hair roots and buccal cells). Complex III deficiency was also demonstrated in these cells. Nevertheless, the mother and the brother were asymptomatic. This mutation had been considered as a cardiomyopathy-generating mutation in a previously reported case, and its pathogenicity has been demonstrated recently in yeast. However, it seems not to fulfil the classical criteria for pathogenicity of a mitochondrial DNA mutation, especially the heteroplasmic status, and to be clinically silent, albeit present, in nonaffected relatives. We suggest that other factors are contributing to the clinical variability expression of the G15498A mtDNA mutation.
Mitochondrial DNA mutations cause disease in >1 in 5000 of the population and approximately 1 in 200 of the population are asymptomatic carriers of a pathogenic mtDNA mutation. Many patients with these pathogenic mtDNA mutations present with a progressive, disabling neurological syndrome that leads to major disability and premature death. There is currently no effective treatment for mitochondrial disorders, placing great emphasis on preventing the transmission of these diseases. An empiric approach can be used to guide genetic counseling for common mtDNA mutations, but many families transmit rare or unique molecular defects. There is therefore a pressing need to develop techniques to prevent transmission based on a solid understanding of the biological mechanisms. Several recent studies have cast new light on the genetics and cell biology of mtDNA inheritance, but these studies have also raised new controversies.
Nuclear proteins that raise mitochondrial mutation rates
The genetic stability of mtDNA in every mammal (indeed every eukaryote) depends critically on the accuracy of dna replication. The consequences of any mutation in this machinery would be greatly amplified (like the broomsticks in the Sorcerer's Apprentice) by subsequent somatic errors created in replicating mitochondrial genomes. It is essential to consider these genes given the apparent elevated rate of mitochondrial polymorphism reported for bison and yak.
The nuclear encoded, mitochondrially functioning dna polymerase POLG on chr 15, the catalytic subunit The catalytic subunit (dna polymerase itself, 3’-5’ exonuclease for proofreading, 5’deoxyribosephosphate lyase for base excision repair), deserves special mention in regards to the extraordinary observed rates of yak and bison coding polymorphisms. Some 90 distinct [human disease alleles are known along the 1239 residue protein, causing progressive external ophthalmoplegia, sensory and ataxic neuropathy, Alpers syndrome, and male infertility (see PEOA1, SANDO, AHS, MNGIE at OMIM). POLG also contains a polyglutamine tract near its N-terminus of length 13 in human that may be subject to polymorphic replication slippage.
POLG is accompanied by an accessory dimer of POLG2. Now receiving considerable attention, two mitochondrial disease alleles have been found, G416A and G451E (causing adPEO). A helicase (PEO1 or twinkle) causing an adult-onset progressive external ophthalmoplegia PEO and topoisomerase TOP1MT are other nuclear encoded proteins critical to mitochondrial dna replication. The latter binds a specific site in the D loop control region. These too have been implicated in rare mitochondrial diseases.
These enzymes, especially POLG, needs extensive sequencing in bison and yak (indeed every once-bottlenecked endangered species). That might done economically on a population scale with whole-exome chips rather than sequencing whole genomes. The POLG gene itself is difficult to study in isolation, being comprised of 23 exons spread out over 18490 bp.
No sequencing of yak or bison POLG has been done yet but that of cow, sheep and pig etc are readily retrieved from their respective genome projects. The Bos taurus POLG protein is 90% identical to human; it has not been specifically studied.
Numts: excluding mitochondrial pseudogenes
Mitochondrial research has been plagued by numt pseudogene alleles mistakenly obtained from the nuclear genome by primer cross-over. Here rna transcribed from mitochondrial genes somehow exits the mitochondria, enters the cell nucleus, gets reverse-transcribed into dna, and then gets heritably integrated into the nuclear junk genome (where it generally is not transcribed and rapidly accrues the mutation pattern of a pseudogene), sometimes becoming fixed across the entire population and even diagnostic of it.
This seemingly implausible sequence of events is surprisingly common. Counts for any species with assembled genome can quickly be conducted by Blat at the UCSC genome browser, though very old events would be missed. Querying cow genome for CYTB nuclear pseudogenes shows 19 nuclear genome matches to cytochrome b, ranging from quite strong to barely significant.
The best match occurs on cow chr28:34924178-34924995. Not quite full length 3', it contains 7 internal stop codons, 52 addition missense mutations (that characteristically do not follow site conservation patterns), and various indels and frameshifts. Not particularly recent, its date of formation could be bracketed by examining sheep and pig genomes for an orthologous numt at syntenic location (+psCYTB +SFTPD (or CGN1, bovine conglutinin).
It's not clear pig contains the orthologous pseudogene at chr14:34281258-34281963 because this feature is not immediately syntenic to porcine SFTPD at chr14:85511174-85522800. If so, the most recent CYTB pseudogene in cow predates the divergence of cow and pig. It then will be found in both yak and bison genomes unless lost through large-scale deletion. Note pig has a much more recent CYTB at chr2:104178282-104179415.
The sheep genome is not currently in a satisfactory state of assembly. This is far more likely than pig to contain a demonstrably syntenic CYTB pseudogene. No sheep pseudogenes are posted at GenBank nr nor locatable by tblastn against wgs or hgts databases. Although 31 CYTB pseudogenes from 11 pecoran species are available, these species all lack genome projects. However upon blastn of the cow chr28 feature, Kobus kob (AF052940) and Capra hircus (GU120393) have very strong matches.
Recent numt pseudogenes can capture ancestral values that prevailed in the mitochondria at the time of formation. Unlike bone fossils, this dna has steadily accrued changes up to the present, but the benefit is nuclear pseudogenes evolve up to 12 times slower than the mitochondrial parental gene. Thus it might represent an atypical heteroplasmic allele existing at that time, be affected by lineage sorting, or reflect a parallel nuclear mutation and so not really settle the issue of ancestral value. A joint tree (1, 2) containing both mitochondrial CYTBs and nuclear pseudogenes (as outgroups) considered in chamois (Rupicapra) has many complexities because genes evolve so differantly in the two compartments (for example the pseudogene might have arisen from a heteroplasmic variant that existed at the time).
The yak study specifically considered whether numts could explain divergent, low-frequency mtDNA haplotypes, but ruled out all but the very most recent on the basis of the separate confirmatory D-loop haplotype phylogeny and great similarity to other haplotypes without unusual sequence features.
Recommendations for bison conservation genomics
(to be continued shortly)
Recommendations for yak conservation genomics
When yak and bison mitochondrial genomes are sequenced and polymorphisms are reported to GenBank, what exactly does that mean? Presumably it reflects an overwhelmingly dominant value of whatever heteroplasmy existed in the tissue sample used to sequence the dna.
The key bison study used white blood cells as dna source, rather than muscle/skin in the yak data. One might imagine this fraction of whole blood is quite heterogeneous in terms of stem cell origin -- five different, diverse leukocyte types exist -- but these all derive from a single hematopoietic stem cell type in bone marrow. Consequently, no other cell types of these bison was sampled. Thus we do not know whether the observed polymorphisms there are heritable (apart from those observed in multiple animals).
Mitochondrial disease in yak is not straightforward to analyze:
"Heteroplasmy is the presence of a mixture of more than one type of an organellar genome within a cell or individual. It is a factor for the severity of mitochondrial diseases, since every eukaryotic cell contains many hundreds of mitochondria with hundreds of copies of mtDNA, it is possible and indeed very frequent for mutations to affect only some of the copies, while the remaining ones are unaffected.
Bovine oocyte mitochondrial issues have been studied for decades, with novel explanations how germline mutations might propagate:
GS Michaels 1982: Restriction endonuclease analysis and direct nucleotide sequencing of bovine mitochondrial DNA have revealed a high apparent rate of sequence divergence between maternally related individuals. Oocytes had 260,000 dna genomic copies per cell, whereas primary bovine tissue culture cells contained only 2,600 copies. These experiments demonstrate directly the amplification of mitochondrial DNA in mammalian oocytes and are consistent with models which could generate mitochondrial DNA polymorphisms by unequal amplification of mitochondrial genomes within an animal.
For yak, since oocytes are not used, the observed polymorphisms are not necessarily heritable even for female individuals (male mitochondria are not passed on). However in the case of yak polymorphisms I118T (domestic) and I192T (wild), multiple individuals (5, 2 respectively) sampled carried the same rare change, strongly implying (unless these are mutational hotspots) that these are entrenched in the germline and so inherited. Oocyte heteroplasmy however is also heritable so wildtype may still persist. The other polymorphisms may be mere somatic mutations that attained abundance in the sampled tissue but are still complemented by residual wildtype. This would have to be pursued in additional tissues or more definitively by sequencing offspring, perhaps not feasible in wild yak.
In summary, even deleterious polymorphisms may have limited effects, depending on stem cell origin and compensation by the wildtype component of heteroplasmy. On the other hand, should a bad alleles exert a negative dominant effect even as the minority allele in the mitochondria in which it resides (eg tainting oligomeric proteins), it would still have a deleterious phenotype even though it never comes to 100% frequency in any particular cell type. Somatic mutations in bison and yak may have limited impacts if onset of disease is delayed to late adulthood as in human. For conservation genomics, we are primarily concerned with heritable mitochondrial mutations, though enhanced levels of somatic mutations (due say to a faulty POLG dna polymerase) are also a concern.
In domestic yak, animals bearing I118T should not be encouraged to reproduce. To be on the safe side, higher frequencies of the other deleterious alleles are also undesirable, even though not quite proven to be heritable.
In wild yak, I192T is the primary cause of concern. It should be avoided if captive breeding comes into play. A017T, D214N, and V329M are not deleterious mutations but rather natural and possibly adaptive parts of yak diversity whose continuation should be encouraged.
These preliminary recommendations are based solely on CYTB. Since only rare recombination occurs in mitochondria (that could bring good alleles on different genes together) and no paternal contribution can dilute out undesirable heteroplasmy, it is unclear how these recommendations can be implemented, much less reconciled from those emerging from independent considerations of the other 12 mitochondrial genes.
Bioinformatic tips and tricks
New sequencing technologies have greatly affected the amount of mammalian mitochondrial genomic data available at GenBank. Five years ago, it was acceptable to publish population-level D loop sequences accompanied by a few fragmentary coding reads; today, a publication might offer 60-70 entire mitochondrial genomes. This favors evolutionary study of mitochondrial proteins over comparative genomics of nuclear genome products because the latter is still restricted to around 50 species (Dec 2010) almost all incompletely sequenced.
Many long-standing issues such as introgression, historic bottlenecks, population mixing, accrual of deleterious coding variants, hard polytomies, and lineage sorting during speciation can now be approached and resolved, especially with the increasing sequencing of end-Pleistocene frozen dna. This may allow more enlightened management of endangered species such as bison where populations reached rock bottom -- recovering numbers is not enough if genomic integrity is still at risk.
However, the flood of data raises significant issues in extraction of significant information: it is not instructive to align the tens of thousands of sequences available for each of 13 mitochondrial proteins -- that give a an intractable array of 3789 amino acids by 12500 sequences, enough to fill 20 x 100 = 2000 screens on the largest possible computer monitor. That data must be distilled down somehow to take-away information.
This section explains a practical desktop protocol for extracting the 'reduced phylogenetic alphabet' at each residue of the mitochondrial proteome. The method depends heavily on current capabilities of Blastp at NCBI and so may not be completely stable to changes made there over time.
First note that tBlastn cannot be used against the nr or wgs nucleotide databases at NCBI (or with Blat at UCSC) since the significantly different genetic code of mammalian mitochondria is no longer supported as a parameter option. Other oddities involve missing terminal nucleotides that are added before translation. However mitochondrial dna is usually translated sensibly at GenBank protein entries.
The vertebrate mitochondrial code:
TTT F Phe TCT S Ser TAT Y Tyr TGT C Cys
TTC F Phe TCC S Ser TAC Y Tyr TGC C Cys
TTA L Leu TCA S Ser TAA * Ter TGA W Trp
TTG L Leu TCG S Ser TAG * Ter TGG W Trp
CTT L Leu CCT P Pro CAT H His CGT R Arg
CTC L Leu CCC P Pro CAC H His CGC R Arg
CTA L Leu CCA P Pro CAA Q Gln CGA R Arg
CTG L Leu CCG P Pro CAG Q Gln CGG R Arg
ATT I Ile ACT T Thr AAT N Asn AGT S Ser
ATC I Ile i ACC T Thr AAC N Asn AGC S Ser
ATA M Met i ACA T Thr AAA K Lys AGA * Ter Bos can use ATA as initiation codon
ATG M Met i ACG T Thr AAG K Lys AGG * Ter
GTT V Val GCT A Ala GAT D Asp GGT G Gly
GTC V Val GCC A Ala GAC D Asp GGC G Gly
GTA V Val GCA A Ala GAA E Glu GGA G Gly
GTG V Val i GCG A Ala GAG E Glu GGG G Gly
AAs = FFLLSSSSYY**CCWWLLLLPPPPHHQQRRRRIIMMTTTTNNKKSS**VVVVAAAADDEEGGGG
Start = --------------------------------MMMM---------------M------------
Base1 = TTTTTTTTTTTTTTTTCCCCCCCCCCCCCCCCAAAAAAAAAAAAAAAAGGGGGGGGGGGGGGGG
Base2 = TTTTCCCCAAAAGGGGTTTTCCCCAAAAGGGGTTTTCCCCAAAAGGGGTTTTCCCCAAAAGGGG
Base3 = TCAGTCAGTCAGTCAGTCAGTCAGTCAGTCAGTCAGTCAGTCAGTCAGTCAGTCAGTCAGTCAG
Blastp output at NCBI now has a very useful feature: clustering of identical individual sequences into single alignments, display of multiplicities, with all the accessions visible with an extra click. The only exception involves double-counting of SwissProt entries which, since SwissProt conducts no sequencing, always arise from another entry.
After collecting high resolution amino acid frequencies at a given site, it is necessary to determine the phylogenetic distribution of each variant (in practice just those of moderate occurrence). That is now very convenient to do provided the associated accessions have been saved:
Simply paste the blastp match list of protein accessions having the chosen amino acid variant into the Entrez text query box. Never mind if it only returns 20 out of your 157 input sequences -- it hasn't forgotten. It doesn't matter if the list has redundant entries (typically SwissProt and the protein giving rise to the SwissProt entry). After retrieval, set the "Find Related Data" to "Taxonomy" and wait for the options to load, then click "Find Items".
Miraculously, this returns a page that can be set to display a text phylogenetic tree your input sequences, the full set entered with all redundancy removed. That text tree has labelled higher taxonomic nodes and individual species deeper down. Final edits can be made quickly that capture the phylogenetic spread of the variant allele for interpretive purposes.
The two most common outcomes:
- all the species carrying the variant comprise a monophyletic clade. If the origin of the clade is fairly ancient, then the variation is a derived informative adaptive change relative to ancestral (synapomorphy). If the site is invariant in all members of the co-clade (meaning the ancestral state has persisted to all other extant species), then the site is a phyloSNP (definition and examples: 1 2 3 4).
- species carrying the variation are scattered incoherently across the mammalian phylogenetic tree. This means that the variation has arisen multiple times (all fairly recently) but has not persisted when it arose earlier, ie it is not a preferred allele for this protein at this site and gets replaced.